Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll-like receptor 4 activation in human macrophages
- PMID: 22194611
- PMCID: PMC3285363
- DOI: 10.1074/jbc.M111.289322
Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll-like receptor 4 activation in human macrophages
Abstract
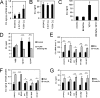
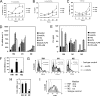
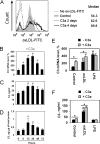
Complement C3 is a pivotal component of three cascades of complement activation. C3 is expressed in human atherosclerotic lesions and is involved in atherogenesis. However, the mechanism of C3 accumulation in atherosclerotic lesions is not well elucidated. We show that acetylated low density lipoprotein and oxidized low density lipoprotein (oxLDL) increase C3 gene expression and protein secretion by human macrophages. Modified LDL (mLDL)-mediated activation of C3 expression mainly depends on liver X receptor (LXR) and partly on Toll-like receptor 4 (TLR4), whereas C3 secretion is increased due to TLR4 activation by mLDL. LXR agonist TO901317 stimulates C3 gene expression in human monocyte-macrophage cells but not in human hepatoma (HepG2) cells. We find LXR-responsive element inside of the promoter region of the human C3 gene, which binds to LXRβ in macrophages but not in HepG2 cells. We show that C3 expression and secretion is decreased in IL-4-treated (M2) and increased in IFNγ/LPS-stimulated (M1) human macrophages as compared with resting macrophages. LXR agonist TO901317 potentiates LPS-induced C3 gene expression and protein secretion in macrophages, whereas oxLDL differently modulates LPS-mediated regulation of C3 in M1 or M2 macrophages. Treatment of human macrophages with anaphylatoxin C3a results in stimulation of C3 transcription and secretion as well as increased oxLDL accumulation and augmented oxLDL-mediated up-regulation of the C3 gene. These data provide a novel mechanism of C3 gene regulation in macrophages and suggest new aspects of cross-talk between mLDL, C3, C3a, and TLR4 during development of atherosclerotic lesions.
Figures




Similar articles
-
Peroxisome proliferator-activated receptor α positively regulates complement C3 expression but inhibits tumor necrosis factor α-mediated activation of C3 gene in mammalian hepatic-derived cells.J Biol Chem. 2013 Jan 18;288(3):1726-38. doi: 10.1074/jbc.M112.437525. Epub 2012 Nov 20. J Biol Chem. 2013. PMID: 23168409 Free PMC article.
-
Regulation of Apolipoprotein A-I Gene Expression in Human Macrophages by Oxidized Low-Density Lipoprotein.Biochemistry (Mosc). 2021 Oct;86(10):1201-1213. doi: 10.1134/S0006297921100047. Biochemistry (Mosc). 2021. PMID: 34903152
-
Phospholipase A(2)-modified low-density lipoprotein activates liver X receptor in human macrophages.Cell Biochem Biophys. 2012 Jun;63(2):143-9. doi: 10.1007/s12013-012-9351-4. Cell Biochem Biophys. 2012. PMID: 22367754
-
The role of the mLDL-induced activation of the complement system classical pathway and C3 expression stimulation in atherosclerosis.Ter Arkh. 2018 Apr 19;90(4):100-104. doi: 10.26442/terarkh2018904100-104. Ter Arkh. 2018. PMID: 30701883 Review.
-
Scientific memory from the early nineties; a common project with professors late János Gergely and Anna Erdei.Biol Futur. 2021 Mar;72(1):3-5. doi: 10.1007/s42977-020-00056-y. Epub 2021 Jan 10. Biol Futur. 2021. PMID: 34554495 Review.
Cited by
-
Preeclampsia and Obesity-The Preventive Role of Exercise.Int J Environ Res Public Health. 2023 Jan 10;20(2):1267. doi: 10.3390/ijerph20021267. Int J Environ Res Public Health. 2023. PMID: 36674022 Free PMC article. Review.
-
Production of complement components by cells of the immune system.Clin Exp Immunol. 2017 May;188(2):183-194. doi: 10.1111/cei.12952. Epub 2017 Mar 24. Clin Exp Immunol. 2017. PMID: 28249350 Free PMC article. Review.
-
Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages.Int J Mol Sci. 2024 Jun 12;25(12):6457. doi: 10.3390/ijms25126457. Int J Mol Sci. 2024. PMID: 38928163 Free PMC article.
-
More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation.Immunol Rev. 2016 Nov;274(1):233-244. doi: 10.1111/imr.12467. Immunol Rev. 2016. PMID: 27782328 Free PMC article. Review.
-
Peroxisome proliferator-activated receptor α positively regulates complement C3 expression but inhibits tumor necrosis factor α-mediated activation of C3 gene in mammalian hepatic-derived cells.J Biol Chem. 2013 Jan 18;288(3):1726-38. doi: 10.1074/jbc.M112.437525. Epub 2012 Nov 20. J Biol Chem. 2013. PMID: 23168409 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

