Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1
- PMID: 22194616
- PMCID: PMC3281598
- DOI: 10.1074/jbc.M111.277731
Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1
Erratum in
- J Biol Chem. 2012 Mar 30;287(14):11283. Yan, Shi Fang [added]
Abstract
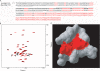
The receptor for advanced glycation end products (RAGE) is a multiligand cell surface macromolecule that plays a central role in the etiology of diabetes complications, inflammation, and neurodegeneration. The cytoplasmic domain of RAGE (C-terminal RAGE; ctRAGE) is critical for RAGE-dependent signal transduction. As the most membrane-proximal event, mDia1 binds to ctRAGE, and it is essential for RAGE ligand-stimulated phosphorylation of AKT and cell proliferation/migration. We show that ctRAGE contains an unusual α-turn that mediates the mDia1-ctRAGE interaction and is required for RAGE-dependent signaling. The results establish a novel mechanism through which an extracellular signal initiated by RAGE ligands regulates RAGE signaling in a manner requiring mDia1.
Figures




References
-
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. (1992) Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267, 14998–15004 - PubMed
-
- Xie J., Reverdatto S., Frolov A., Hoffmann R., Burz D. S., Shekhtman A. (2008) Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 283, 27255–27269 - PubMed
-
- Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M. F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A. M. (1999) RAGE mediates a novel proinflammatory axis. A central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 - PubMed
-
- Hori O., Yan S. D., Ogawa S., Kuwabara K., Matsumoto M., Stern D., Schmidt A. M. (1996) The receptor for advanced glycation end-products has a central role in mediating the effects of advanced glycation end-products on the development of vascular disease in diabetes mellitus. Nephrol. Dial. Transplant 11, 13–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

