Human mitochondrial DNA polymerase γ exhibits potential for bypass and mutagenesis at UV-induced cyclobutane thymine dimers
- PMID: 22194617
- PMCID: PMC3308766
- DOI: 10.1074/jbc.M111.306852
Human mitochondrial DNA polymerase γ exhibits potential for bypass and mutagenesis at UV-induced cyclobutane thymine dimers
Abstract
Cyclobutane thymine dimers (T-T) comprise the majority of DNA damage caused by short wavelength ultraviolet radiation. These lesions generally block replicative DNA polymerases and are repaired by nucleotide excision repair or bypassed by translesion polymerases in the nucleus. Mitochondria lack nucleotide excision repair, and therefore, it is important to understand how the sole mitochondrial DNA polymerase, pol γ, interacts with irreparable lesions such as T-T. We performed in vitro DNA polymerization assays to measure the kinetics of incorporation opposite the lesion and bypass of the lesion by pol γ with a dimer-containing template. Exonuclease-deficient pol γ bypassed thymine dimers with low relative efficiency; bypass was attenuated but still detectable when using exonuclease-proficient pol γ. When bypass did occur, pol γ misincorporated a guanine residue opposite the 3'-thymine of the dimer only 4-fold less efficiently than it incorporated an adenine. Surprisingly, the pol γ exonuclease-proficient enzyme excised the incorrectly incorporated guanine at similar rates irrespective of the nature of the thymines in the template. In the presence of all four dNTPs, pol γ extended the primer after incorporation of two adenines opposite the lesion with relatively higher efficiency compared with extension past either an adenine or a guanine incorporated opposite the 3'-thymine of the T-T. Our results suggest that T-T usually stalls mitochondrial DNA replication but also suggest a mechanism for the introduction of point mutations and deletions in the mitochondrial genomes of chronically UV-exposed cells.
Figures

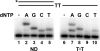
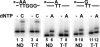

Similar articles
-
Translesion synthesis past acrolein-derived DNA adducts by human mitochondrial DNA polymerase γ.J Biol Chem. 2013 May 17;288(20):14247-14255. doi: 10.1074/jbc.M113.458802. Epub 2013 Mar 30. J Biol Chem. 2013. PMID: 23543747 Free PMC article.
-
DNA polymerase iota-dependent translesion replication of uracil containing cyclobutane pyrimidine dimers.DNA Repair (Amst). 2006 Feb 3;5(2):210-8. doi: 10.1016/j.dnarep.2005.09.011. Epub 2005 Nov 2. DNA Repair (Amst). 2006. PMID: 16263340
-
Mutational specificity of gamma-radiation-induced guanine-thymine and thymine-guanine intrastrand cross-links in mammalian cells and translesion synthesis past the guanine-thymine lesion by human DNA polymerase eta.Biochemistry. 2008 Aug 5;47(31):8070-9. doi: 10.1021/bi800529f. Epub 2008 Jul 11. Biochemistry. 2008. PMID: 18616294 Free PMC article.
-
[Mechanisms of targeted frameshift mutations--insertion formation under error-prone or SOS synthesis of DNA containing CIS-SYN cyncyclobutane thymine dimers].Mol Biol (Mosk). 2014 Jul-Aug;48(4):531-42. Mol Biol (Mosk). 2014. PMID: 25842840 Review. Russian.
-
Mass Spectrometry-Based Quantitative Strategies for Assessing the Biological Consequences and Repair of DNA Adducts.Acc Chem Res. 2016 Feb 16;49(2):205-13. doi: 10.1021/acs.accounts.5b00437. Epub 2016 Jan 13. Acc Chem Res. 2016. PMID: 26758048 Free PMC article. Review.
Cited by
-
Mitochondrial DNA damage induced autophagy, cell death, and disease.Front Biosci (Landmark Ed). 2016 Jan 1;21(1):42-54. doi: 10.2741/4375. Front Biosci (Landmark Ed). 2016. PMID: 26709760 Free PMC article. Review.
-
Mitochondria as a target of environmental toxicants.Toxicol Sci. 2013 Jul;134(1):1-17. doi: 10.1093/toxsci/kft102. Epub 2013 Apr 29. Toxicol Sci. 2013. PMID: 23629515 Free PMC article. Review.
-
Programmed mitophagy at the oocyte-to-zygote transition promotes species immortality.Res Sq [Preprint]. 2025 Apr 9:rs.3.rs-6330979. doi: 10.21203/rs.3.rs-6330979/v1. Res Sq. 2025. PMID: 40297685 Free PMC article. Preprint.
-
Plant organellar DNA polymerases are replicative and translesion DNA synthesis polymerases.Nucleic Acids Res. 2017 Oct 13;45(18):10751-10763. doi: 10.1093/nar/gkx744. Nucleic Acids Res. 2017. PMID: 28977655 Free PMC article.
-
Mitophagy and DNA damage signaling in human aging.Mech Ageing Dev. 2020 Mar;186:111207. doi: 10.1016/j.mad.2020.111207. Epub 2020 Jan 7. Mech Ageing Dev. 2020. PMID: 31923475 Free PMC article. Review.
References
-
- Friedberg E. C., Walker G. C., Siede W., Wood R. D., Schultz R. A., Ellenberger T. (2006) DNA Repair and Mutagenesis, 2nd Ed., pp. 29–31, American Society for Microbiology, Washington, D. C.
-
- Hayes F. N., Williams D. L., Ratlift R. L., Varghese A. J., Rupert C. S. (1971) Effect of a single thymine photodimer on the oligodeoxythymidylate-polydeoxyadenylate interaction. J. Am. Chem. Soc. 93, 4940–4942 - PubMed
-
- Pfeifer G. P., You Y. H., Besaratinia A. (2005) Mutations induced by ultraviolet light. Mutat. Res. 571, 19–31 - PubMed
-
- Bootsma D., Humphrey R. M. (1968) The progression of mammalian cells through the division cycle following ultraviolet irradiation. Mutat. Res. 5, 289–298 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

