The effect of the interaction between aquaporin 0 (AQP0) and the filensin tail region on AQP0 water permeability
- PMID: 22194645
- PMCID: PMC3244488
The effect of the interaction between aquaporin 0 (AQP0) and the filensin tail region on AQP0 water permeability
Abstract
Purpose: To study the interaction between the lens-specific water channel protein, aquaporin 0 (AQP0) and the lens-specific intermediate filament protein, filensin, and the effect of this interaction on the water permeability of AQP0. The effect of other factors on the interaction was also investigated.
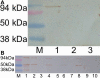
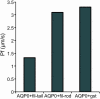
Methods: Expression plasmids were constructed in which glutathione-S-transferase (GST) was fused to the AQP0 COOH-terminal region (GST-AQP0-C), which contains the major phosphorylation sites of the protein. Plasmids for AQP0 COOH-terminal mutants were also constructed in which one, three or five sites were pseudophosphorylated, and the proteins expressed from these GST-fusion plasmids were assayed for their interaction with lens proteins. Expressed recombinant GST-fusion proteins were purified using glutathione beads and incubated with rat lens extract. Western blotting was used to identify the lens proteins that interacted with the GST-fusion proteins. Filensin tail and rod domains were also expressed as GST-fusion proteins and their interactions with AQPO were analyzed. Additionally, the water permeability of AQP0 was calculated by expressing AQP0 with or without the filensin peptide on the cell membrane of Xenopus oocytes by injecting cRNAs for AQP0 and filensin.
Results: The GST-AQP0-C construct interacted with the tail region of lens filensin and the GST-filensin-tail construct interacted with lens AQP0, but the GST-filensin-rod construct did not interact with AQP0. GST-AQP0-C also interacted with a purified recombinant filensin-tail peptide after cleavage from GST. The AQP0/filensin-tail interaction was not affected by pseudophosphorylation of the AQP0 COOH-terminal tail, nor was it affected by changes in pH. Xenopus oocytes expressing AQP0 on the plasma membrane showed increased water permeability, which was lowered when the filensin COOH-terminal peptide cRNA was coinjected with the cRNA for AQP0.
Conclusions: The filensin COOH-terminal tail region interacted with the AQP0 COOH-terminal region and the results strongly suggested that the interaction was direct. It appears that interactions between AQP0 and filensin helps to regulate the water permeability of AQP0 and to organize the structure of lens fiber cells, and may also help to maintain the transparency of the lens.
Figures



References
-
- Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta. 2009;1788:1213–28. - PubMed
-
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–6. - PubMed
-
- Iandiev I, Pannicke T, Härtig W, Grosche J, Wiedemann P, Reichenbach A, Bringmann A. Localization of aquaproin-0 immunoreactivity in the rat retina. Neurosci Lett. 2007;426:81–6. - PubMed
-
- Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710–7. - PubMed
-
- Alcalá J, Lieska N, Maisel H. Protein composition of bovine lens cortical fiber cell membranes. Exp Eye Res. 1975;21:581–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
