Plasticity of BRCA2 function in homologous recombination: genetic interactions of the PALB2 and DNA binding domains
- PMID: 22194698
- PMCID: PMC3240595
- DOI: 10.1371/journal.pgen.1002409
Plasticity of BRCA2 function in homologous recombination: genetic interactions of the PALB2 and DNA binding domains
Abstract
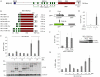
The breast cancer suppressor BRCA2 is essential for the maintenance of genomic integrity in mammalian cells through its role in DNA repair by homologous recombination (HR). Human BRCA2 is 3,418 amino acids and is comprised of multiple domains that interact with the RAD51 recombinase and other proteins as well as with DNA. To gain insight into the cellular function of BRCA2 in HR, we created fusions consisting of various BRCA2 domains and also introduced mutations into these domains to disrupt specific protein and DNA interactions. We find that a BRCA2 fusion peptide deleted for the DNA binding domain and active in HR is completely dependent on interaction with the PALB2 tumor suppressor for activity. Conversely, a BRCA2 fusion peptide deleted for the PALB2 binding domain is dependent on an intact DNA binding domain, providing a role for this conserved domain in vivo; mutagenesis suggests that both single-stranded and double-stranded DNA binding activities in the DNA binding domain are required for its activity. Given that PALB2 itself binds DNA, these results suggest alternative mechanisms to deliver RAD51 to DNA. In addition, the BRCA2 C terminus contains both RAD51-dependent and -independent activities which are essential to HR in some contexts. Finally, binding the small peptide DSS1 is essential for activity when its binding domain is present, but not when it is absent. Our results reveal functional redundancy within the BRCA2 protein and emphasize the plasticity of this large protein built for optimal HR function in mammalian cells. The occurrence of disease-causing mutations throughout BRCA2 suggests sub-optimal HR from a variety of domain modulations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

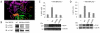

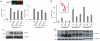
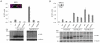


References
-
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. - PubMed
-
- Mimitou EP, Symington LS. Nucleases and helicases take center stage in homologous recombination. Trends Biochem Sci. 2009;34:264–272. - PubMed
-
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 Is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. - PubMed
-
- Chen CF, Chen PL, Zhong Q, Sharp ZD, Lee WH. Expression of BRC repeats in breast cancer cells disrupts the BRCA2-Rad51 complex and leads to radiation hypersensitivity and loss of G(2)/M checkpoint control. J Biol Chem. 1999;274:32931–32935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

