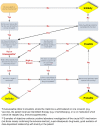
Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool
- PMID: 22194808
- PMCID: PMC3237416
- DOI: 10.1371/journal.pone.0028096
Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool
Abstract
Aim: To develop and test a new adverse drug reaction (ADR) causality assessment tool (CAT).
Methods: A comparison between seven assessors of a new CAT, formulated by an expert focus group, compared with the Naranjo CAT in 80 cases from a prospective observational study and 37 published ADR case reports (819 causality assessments in total).
Main outcome measures: Utilisation of causality categories, measure of disagreements, inter-rater reliability (IRR).
Results: The Liverpool ADR CAT, using 40 cases from an observational study, showed causality categories of 1 unlikely, 62 possible, 92 probable and 125 definite (1, 62, 92, 125) and 'moderate' IRR (kappa 0.48), compared to Naranjo (0, 100, 172, 8) with 'moderate' IRR (kappa 0.45). In a further 40 cases, the Liverpool tool (0, 66, 81, 133) showed 'good' IRR (kappa 0.6) while Naranjo (1, 90, 185, 4) remained 'moderate'.
Conclusion: The Liverpool tool assigns the full range of causality categories and shows good IRR. Further assessment by different investigators in different settings is needed to fully assess the utility of this tool.
Conflict of interest statement
Figures
Similar articles
-
Development of the Liverpool Adverse Drug Reaction Avoidability Assessment Tool.PLoS One. 2017 Jan 3;12(1):e0169393. doi: 10.1371/journal.pone.0169393. eCollection 2017. PLoS One. 2017. PMID: 28046035 Free PMC article.
-
Adverse drug reaction causality assessment tools for drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: room for improvement.Eur J Clin Pharmacol. 2019 Aug;75(8):1135-1141. doi: 10.1007/s00228-019-02670-9. Epub 2019 Mar 27. Eur J Clin Pharmacol. 2019. PMID: 30918988 Free PMC article.
-
Comparison of the Liverpool Causality Assessment Tool vs. the Naranjo Scale for predicting the likelihood of an adverse drug reaction: A retrospective cohort study.Br J Clin Pharmacol. 2023 Aug;89(8):2407-2412. doi: 10.1111/bcp.15704. Epub 2023 Mar 22. Br J Clin Pharmacol. 2023. PMID: 36849649
-
A scoping review of the clinical utility of adverse drug reaction causality analysis tools for use in the hospital setting.Expert Rev Clin Pharmacol. 2024 Dec;17(12):1127-1148. doi: 10.1080/17512433.2024.2429677. Epub 2024 Nov 22. Expert Rev Clin Pharmacol. 2024. PMID: 39535130
-
Toward Kidney-Specific Causality Assessment Tool.Clin Ther. 2022 Jul;44(7):e59-e75. doi: 10.1016/j.clinthera.2022.05.008. Epub 2022 Jun 18. Clin Ther. 2022. PMID: 35725506 Review.
Cited by
-
Comparison of three methods (an updated logistic probabilistic method, the Naranjo and Liverpool algorithms) for the evaluation of routine pharmacovigilance case reports using consensual expert judgement as reference.Drug Saf. 2013 Oct;36(10):1033-44. doi: 10.1007/s40264-013-0083-1. Drug Saf. 2013. PMID: 23828659 Clinical Trial.
-
Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions.Saudi Pharm J. 2016 Jul;24(4):485-93. doi: 10.1016/j.jsps.2015.01.010. Epub 2015 Jan 10. Saudi Pharm J. 2016. PMID: 27330379 Free PMC article. Review.
-
Patients' Identification, Management and Prevention of Adverse Drug Reactions: A Cross-Sectional Survey of Patients with Severe Adverse Drug Reactions.J Clin Med. 2024 Jul 16;13(14):4165. doi: 10.3390/jcm13144165. J Clin Med. 2024. PMID: 39064204 Free PMC article.
-
Causality, Severity, Preventability and Predictability Assessments Scales for Adverse Drug Reactions: A Review.Cureus. 2024 May 9;16(5):e59975. doi: 10.7759/cureus.59975. eCollection 2024 May. Cureus. 2024. PMID: 38854273 Free PMC article. Review.
-
Recurring Fatigue After Biologic Administration: Patient-Reported Data from the Dutch Biologic Monitor.BioDrugs. 2023 Jul;37(4):541-550. doi: 10.1007/s40259-023-00592-8. Epub 2023 Apr 3. BioDrugs. 2023. PMID: 37010772 Free PMC article.
References
-
- Clavenna A, Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–728. - PubMed
-
- Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31:21–37. - PubMed
-
- Turner WM. The Food and Drug Administration algorithm. Special workshop–regulatory. Drug Information Journal. 1984;18:259–266. - PubMed
-
- Arimone Y, Bidault I, Collignon AE, Dutertre JP, Gerardin M, et al. Updating of the French causality assessment method: 29. Fundamental & Clinical Pharmacology. 2010;6
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous