Cultivation of human corneal endothelial cells isolated from paired donor corneas
- PMID: 22194824
- PMCID: PMC3241625
- DOI: 10.1371/journal.pone.0028310
Cultivation of human corneal endothelial cells isolated from paired donor corneas
Abstract
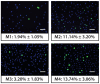
Consistent expansion of human corneal endothelial cells (hCECs) is critical in the development of tissue engineered endothelial constructs. However, a wide range of complex culture media, developed from different basal media have been reported in the propagation of hCECs, some with more success than others. These results are further confounded by donor-to-donor variability. The aim of this study is to evaluate four culture media in the isolation and propagation of hCECs isolated from a series of paired donor corneas in order to negate donor variability. Isolated primary hCECs were cultured in four previously published medium coded in this study as: M1-DMEM; M2-OptiMEM-I; M3-DMEM/F12, & M4-Ham's F12/M199. Primary hCECs established in these conditions were expanded for two passages and analyzed for (1) their propensity to adhere and proliferate; (2) their expression of characteristic corneal endothelium markers: Na+K+/ATPase and ZO-1; and (3) their cellular morphology throughout the study. We found that hCECs isolated in all four media showed rapid attachment when cultured on FNC-coated dishes. However, hCECs established in the four media exhibited different proliferation profiles with striking morphological differences. Corneal endothelial cells cultured in M1 and M3 could not be propagated beyond the first and second passage respectively. The hCECs cultured in M2 and M4 were significantly more proliferative and expressed markers characteristics of human corneal endothelium: Na+K+/ATPase and ZO-1. However, the unique morphological characteristics of cultivated hCECs were not maintained in either M2 or M4 beyond the third passage.The proliferative capacity and morphology of hCECs are vastly affected by the four culture media. For the development of tissue engineered graft materials using cultured hCECs derived from the isolation methodology described in this study, we propose the use of proliferative media M2 or M4 up to the third passage, or before the cultured hCECs lose their unique cellular morphology.
Conflict of interest statement
Figures





References
-
- Peh GS, Beuerman RW, Colman A, Tan DT, Mehta JS. Human Corneal Endothelial Cell Expansion for Corneal Endothelium Transplantation: An Overview. Transplantation. 2011;91:811–819. - PubMed
-
- Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–782. - PubMed
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389. - PubMed
-
- Kaufman HE, Katz JI. Pathology of the corneal endothelium. Invest Ophthalmol Vis Sci. 1977;16:265–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

