PEGylation potentiates the effectiveness of an antagonistic peptide that targets the EphB4 receptor with nanomolar affinity
- PMID: 22194865
- PMCID: PMC3237458
- DOI: 10.1371/journal.pone.0028611
PEGylation potentiates the effectiveness of an antagonistic peptide that targets the EphB4 receptor with nanomolar affinity
Abstract
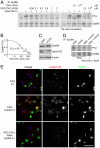
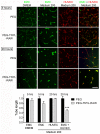
The EphB4 receptor tyrosine kinase together with its preferred ligand, ephrin-B2, regulates a variety of physiological and pathological processes, including tumor progression, pathological forms of angiogenesis, cardiomyocyte differentiation and bone remodeling. We previously reported the identification of TNYL-RAW, a 15 amino acid-long peptide that binds to the ephrin-binding pocked of EphB4 with low nanomolar affinity and inhibits ephrin-B2 binding. Although ephrin-B2 interacts promiscuously with all the EphB receptors, the TNYL-RAW peptide is remarkably selective and only binds to EphB4. Therefore, this peptide is a useful tool for studying the biological functions of EphB4 and for imaging EphB4-expressing tumors. Furthermore, TNYL-RAW could be useful for treating pathologies involving EphB4-ephrin-B2 interaction. However, the peptide has a very short half-life in cell culture and in the mouse blood circulation due to proteolytic degradation and clearance by the kidneys and reticuloendothelial system. To overcome these limitations, we have modified TNYL-RAW by fusion with the Fc portion of human IgG1, complexation with streptavidin or covalent coupling to a 40 KDa branched polyethylene glycol (PEG) polymer. These modified forms of TNYL-RAW all have greatly increased stability in cell culture, while retaining high binding affinity for EphB4. Furthermore, PEGylation most effectively increases peptide half-life in vivo. Consistent with increased stability, submicromolar concentrations of PEGylated TNYL-RAW effectively impair EphB4 activation by ephrin-B2 in cultured B16 melanoma cells as well as capillary-like tube formation and capillary sprouting in co-cultures of endothelial and epicardial mesothelial cells. Therefore, PEGylated TNYL-RAW may be useful for inhibiting pathological forms of angiogenesis through a novel mechanism involving disruption of EphB4-ephrin-B2 interactions between endothelial cells and supporting perivascular mesenchymal cells. Furthermore, the PEGylated peptide is suitable for other cell culture and in vivo applications requiring prolonged EphB4 receptor targeting.
Conflict of interest statement
Figures





Similar articles
-
Design, synthesis and characterization of novel small molecular inhibitors of ephrin-B2 binding to EphB4.Biochem Pharmacol. 2013 Feb 15;85(4):507-13. doi: 10.1016/j.bcp.2012.12.005. Epub 2012 Dec 17. Biochem Pharmacol. 2013. PMID: 23253822 Free PMC article.
-
111In-Labeled-TNYLFSPNGPIARAW (TNYL-RAW)-polyethylene glycol–coated core-cross-linked polymeric micelle-Cy7.2011 Sep 18 [updated 2012 Jan 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Sep 18 [updated 2012 Jan 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22279635 Free Books & Documents. Review.
-
Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation.J Bone Miner Res. 2014 Aug;29(8):1900-13. doi: 10.1002/jbmr.2196. J Bone Miner Res. 2014. PMID: 24677183 Free PMC article.
-
64Cu-Tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid-TNYLFSPNGPIARAW (TNYL-RAW).2011 Sep 10 [updated 2011 Dec 15]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Sep 10 [updated 2011 Dec 15]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22206105 Free Books & Documents. Review.
-
In vivo small-animal PET/CT of EphB4 receptors using 64Cu-labeled peptide.J Nucl Med. 2011 Feb;52(2):241-8. doi: 10.2967/jnumed.110.081943. Epub 2011 Jan 13. J Nucl Med. 2011. PMID: 21233177
Cited by
-
Fibronectin Patches as Anchoring Points for Force Sensing and Transmission in Human Induced Pluripotent Stem Cell-Derived Pericytes.Stem Cell Reports. 2020 Jun 9;14(6):1107-1122. doi: 10.1016/j.stemcr.2020.05.001. Epub 2020 May 28. Stem Cell Reports. 2020. PMID: 32470326 Free PMC article.
-
Eph-Ephrin Signaling Mediates Cross-Talk Within the Bone Microenvironment.Front Cell Dev Biol. 2021 Feb 9;9:598612. doi: 10.3389/fcell.2021.598612. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634116 Free PMC article. Review.
-
Inhibition of Eph receptor-ephrin ligand interaction by tea polyphenols.Pharmacol Res. 2012 Oct;66(4):363-73. doi: 10.1016/j.phrs.2012.05.010. Epub 2012 Jun 28. Pharmacol Res. 2012. PMID: 22750215 Free PMC article.
-
HTS by NMR of combinatorial libraries: a fragment-based approach to ligand discovery.Chem Biol. 2013 Jan 24;20(1):19-33. doi: 10.1016/j.chembiol.2012.10.015. Chem Biol. 2013. PMID: 23352136 Free PMC article.
-
Protein-Protein Interaction Inhibitors Targeting the Eph-Ephrin System with a Focus on Amino Acid Conjugates of Bile Acids.Pharmaceuticals (Basel). 2022 Jan 24;15(2):137. doi: 10.3390/ph15020137. Pharmaceuticals (Basel). 2022. PMID: 35215250 Free PMC article. Review.
References
-
- Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. - PubMed
-
- Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–3745. - PubMed
-
- Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

