FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation
- PMID: 22194867
- PMCID: PMC3237475
- DOI: 10.1371/journal.pone.0028614
FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation
Abstract
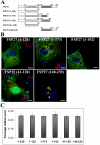
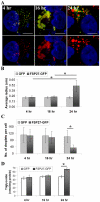
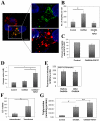
Fat Specific Protein 27 (FSP27), a lipid droplet (LD) associated protein in adipocytes, regulates triglyceride (TG) storage. In the present study we demonstrate that FSP27 plays a key role in LD morphology to accumulate TGs. We show here that FSP27 promotes clustering of the LDs which is followed by their fusion into fewer and enlarged droplets. To map the domains of FSP27 responsible for these events, we generated GFP-fusion constructs of deletion mutants of FSP27. Microscopic analysis revealed that amino acids 173-220 of FSP27 are necessary and sufficient for both the targeting of FSP27 to LDs and the initial clustering of the droplets. Amino acids 120-140 are essential but not sufficient for LD enlargement, whereas amino acids 120-210 are necessary and sufficient for both clustering and fusion of LDs to form enlarged droplets. In addition, we found that FSP27-mediated enlargement of LDs, but not their clustering, is associated with triglyceride accumulation. These results suggest a model in which FSP27 facilitates LD clustering and then promotes their fusion to form enlarged droplets in two discrete, sequential steps, and a subsequent triglyceride accumulation.
Conflict of interest statement
Figures





References
-
- Beckman M. Cell biology. Great balls of fat. Science. 2006;311:1232–1234. - PubMed
-
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. - PubMed
-
- Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. - PubMed
-
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

