Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat
- PMID: 22194894
- PMCID: PMC3237485
- DOI: 10.1371/journal.pone.0028717
Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat
Abstract
Background & aims: Secretin induces bicarbonate-rich hydrocholeresis in healthy individuals, but not in untreated patients with primary biliary cirrhosis (PBC). Ursodeoxycholic acid (UDCA)--the first choice treatment for PBC--restores the secretin response. Compared with humans, secretin has poor effect in experimental normal-rat models with biliary drainage, although it may elicit hydrocholeresis when the bile-acid pool is maintained. In view of the benefits of UDCA in PBC, we used normal-rat models to unravel the acute contribution of UDCA (and/or taurine-conjugated TUDCA) for eliciting the biliary secretin response.
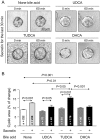
Methods: Intravascular and/or intrabiliary administration of agonists and inhibitors was performed in normal rats with biliary monitoring. Secretin/bile-acid interplay was analyzed in 3D cultured rat cholangiocytes that formed expansive cystic structures with intralumenal hydroionic secretion.
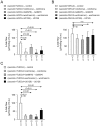
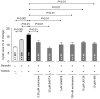
Results: In vivo, secretin stimulates hydrocholeresis upon UDCA/TUDCA infusion, but does not modify the intrinsic hypercholeretic effect of dehydrocholic acid (DHCA). The former effect is dependent on microtubule polymerization, and involves PKCα, PI3K and MEK pathways, as shown by colchicine (i.p.) and retrograde biliary inhibitors. In vitro, while secretin alone accelerates the spontaneous expansion of 3D-cystic structures, this effect is enhanced in the presence of TUDCA, but not UDCA or DHCA. Experiments with inhibitors and Ca(2+)-chelator confirmed that the synergistic effect of secretin plus TUDCA involves microtubules, intracellular Ca(2+), PKCα, PI3K, PKA and MEK pathways. Gene silencing also demonstrated the involvement of the bicarbonate extruder Ae2.
Conclusions: UDCA is conjugated in order to promote secretin-stimulated hydrocholeresis in rats through Ae2, microtubules, intracellular Ca(2+), PKCα, PI3K, PKA, and MEK.
Conflict of interest statement
Figures



References
-
- Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–625. - PubMed
-
- Banales JM, Arenas F, Rodríguez-Ortigosa CM, Sáez E, Uriarte I, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. - PubMed
-
- Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, et al. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413–20419. - PubMed
-
- Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

