Quantification bias caused by plasmid DNA conformation in quantitative real-time PCR assay
- PMID: 22194997
- PMCID: PMC3237602
- DOI: 10.1371/journal.pone.0029101
Quantification bias caused by plasmid DNA conformation in quantitative real-time PCR assay
Abstract
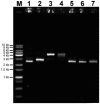
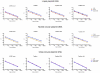
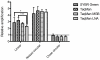
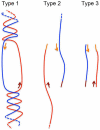
Quantitative real-time PCR (qPCR) is the gold standard for the quantification of specific nucleic acid sequences. However, a serious concern has been revealed in a recent report: supercoiled plasmid standards cause significant over-estimation in qPCR quantification. In this study, we investigated the effect of plasmid DNA conformation on the quantification of DNA and the efficiency of qPCR. Our results suggest that plasmid DNA conformation has significant impact on the accuracy of absolute quantification by qPCR. DNA standard curves shifted significantly among plasmid standards with different DNA conformations. Moreover, the choice of DNA measurement method and plasmid DNA conformation may also contribute to the measurement error of DNA standard curves. Due to the multiple effects of plasmid DNA conformation on the accuracy of qPCR, efforts should be made to assure the highest consistency of plasmid standards for qPCR. Thus, we suggest that the conformation, preparation, quantification, purification, handling, and storage of standard plasmid DNA should be described and defined in the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) to assure the reproducibility and accuracy of qPCR absolute quantification.
Conflict of interest statement
Figures


References
-
- Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. - PubMed
-
- Holst-Jensen A, Ronning SB, Lovseth A, Berdal KG. PCR technology for screening and quantification of genetically modified organisms (GMOs). Anal Bioanal Chem. 2003;375:985–993. - PubMed
-
- Hou Y, Zhang H, Miranda L, Lin S. Serious overestimation in quantitative PCR by circular (supercoiled) plasmid standard: microalgal pcna as the model gene. PLoS One. 2010;5:e9545. doi: 10.1371/journal.pone.0009545. - DOI - PMC - PubMed
-
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

