GmFT2a, a soybean homolog of FLOWERING LOCUS T, is involved in flowering transition and maintenance
- PMID: 22195028
- PMCID: PMC3237611
- DOI: 10.1371/journal.pone.0029238
GmFT2a, a soybean homolog of FLOWERING LOCUS T, is involved in flowering transition and maintenance
Abstract
Background: Flowering reversion can be induced in soybean (Glycine max L. Merr.), a typical short-day (SD) dicot, by switching from SD to long-day (LD) photoperiods. This process may involve florigen, putatively encoded by FLOWERING LOCUS T (FT) in Arabidopsis thaliana. However, little is known about the potential function of soybean FT homologs in flowering reversion.
Methods: A photoperiod-responsive FT homologue GmFT (renamed as GmFT2a hereafter) was cloned from the photoperiod-sensitive cultivar Zigongdongdou. GmFT2a gene expression under different photoperiods was analyzed by real-time quantitative PCR. In situ hybridization showed direct evidence for its expression during flowering-related processes. GmFT2a was shown to promote flowering using transgenic studies in Arabidopsis and soybean. The effects of photoperiod and temperature on GmFT2a expression were also analyzed in two cultivars with different photoperiod-sensitivities.
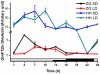
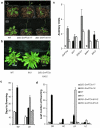
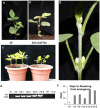
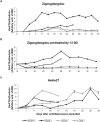
Results: GmFT2a expression is regulated by photoperiod. Analyses of GmFT2a transcripts revealed a strong correlation between GmFT2a expression and flowering maintenance. GmFT2a transcripts were observed continuously within the vascular tissue up to the shoot apex during flowering. By contrast, transcripts decreased to undetectable levels during flowering reversion. In grafting experiments, the early-flowering, photoperiod-insensitive stock Heihe27 promotes the appearance of GmFT2a transcripts in the shoot apex of scion Zigongdongdou under noninductive LD conditions. The photothermal effects of GmFT2a expression diversity in cultivars with different photoperiod-sensitivities and a hypothesis is proposed.
Conclusion: GmFT2a expression is associated with flowering induction and maintenance. Therefore, GmFT2a is a potential target gene for soybean breeding, with the aim of increasing geographic adaptation of this crop.
Conflict of interest statement
Figures
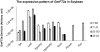



References
-
- Bäurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. - PubMed
-
- Bernier G, Périlleux C. A physiological overview of the genetics of flowering time control. Plant Biotechnol J. 2005;3:3–16. - PubMed
-
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. - PubMed
-
- Kobayashi Y, Weigel D. Move on up, it's time for change—mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

