LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor
- PMID: 22195035
- PMCID: PMC3237609
- DOI: 10.1371/journal.pone.0029260
LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor
Abstract
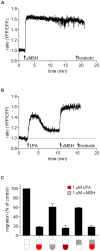
Lysophosphatidic acid (LPA), a lipid mediator enriched in serum, stimulates cell migration, proliferation and other functions in many cell types. LPA acts on six known G protein-coupled receptors, termed LPA(1-6), showing both overlapping and distinct signaling properties. Here we show that, unexpectedly, LPA and serum almost completely inhibit the transwell migration of B16 melanoma cells, with alkyl-LPA(18:1) being 10-fold more potent than acyl-LPA(18:1). The anti-migratory response to LPA is highly polarized and dependent on protein kinase A (PKA) but not Rho kinase activity; it is associated with a rapid increase in intracellular cAMP levels and PIP3 depletion from the plasma membrane. B16 cells express LPA(2), LPA(5) and LPA(6) receptors. We show that LPA-induced chemorepulsion is mediated specifically by the alkyl-LPA-preferring LPA(5) receptor (GPR92), which raises intracellular cAMP via a noncanonical pathway. Our results define LPA(5) as an anti-migratory receptor and they implicate the cAMP-PKA pathway, along with reduced PIP3 signaling, as an effector of chemorepulsion in B16 melanoma cells.
Conflict of interest statement
Figures
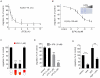

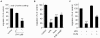
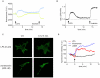
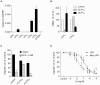

References
-
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. - PubMed
-
- van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. - PubMed
-
- Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

