Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin
- PMID: 22195992
- PMCID: PMC6837545
- DOI: 10.1179/1351000211Y.0000000015
Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin
Erratum in
- Redox Rep. 2012;17(4):180
Abstract
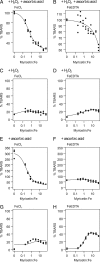
Objectives: Flavonoids are ubiquitous phenolic plant metabolites. Many of them are well known for their pro- and antioxidant properties. Myricetin has been reported to be either a potent antioxidant or a pro-oxidant depending on the conditions. The reaction conditions for the pro- and antioxidant activities were therefore investigated using variations of the deoxyribose degradation assay systems.
Methods: The deoxyribose degradation assay systems were conducted as follows; H(2)O(2)/Fe(III)/ascorbic acid, H(2)O(2)/Fe(III), Fe(III)/ascorbic acid, and Fe(III). Each system was carried out in two variants, FeCl(3) (iron ions added as FeCl(3)) and FeEDTA (iron added in complex with ethylenediaminetetraacetic acid).
Results: When ascorbic acid was present, myricetin showed antioxidant properties, especially when it occurred in complex with iron. In ascorbic acid-free systems, pro-oxidant activities prevailed, which where enhanced if iron was in complex with EDTA.
Discussion: Myricetin's antioxidant activity depends on both the reactive oxygen species (ROS) scavenging and iron ions chelation properties. The pro-oxidative properties are caused by reduction of molecular oxygen to ROS and iron(III) to iron(II). Myricetin is able to substitute for ascorbic acid albeit less efficiently.
Figures



Similar articles
-
In Vitro Evaluation of Pro- and Antioxidant Effects of Flavonoid Tricetin in Comparison to Myricetin.Molecules. 2020 Dec 11;25(24):5850. doi: 10.3390/molecules25245850. Molecules. 2020. PMID: 33322312 Free PMC article.
-
Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions.Basic Clin Pharmacol Toxicol. 2005 May;96(5):352-60. doi: 10.1111/j.1742-7843.2005.pto_03.x. Basic Clin Pharmacol Toxicol. 2005. PMID: 15853927
-
Dual mechanism of mangiferin protection against iron-induced damage to 2-deoxyribose and ascorbate oxidation.Pharmacol Res. 2006 Mar;53(3):253-60. doi: 10.1016/j.phrs.2005.06.006. Epub 2006 Jan 10. Pharmacol Res. 2006. PMID: 16412661
-
Antioxidants, oxidative damage and oxygen deprivation stress: a review.Ann Bot. 2003 Jan;91 Spec No(2):179-94. doi: 10.1093/aob/mcf118. Ann Bot. 2003. PMID: 12509339 Free PMC article. Review.
-
Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond.Int J Mol Sci. 2021 Jan 11;22(2):646. doi: 10.3390/ijms22020646. Int J Mol Sci. 2021. PMID: 33440733 Free PMC article. Review.
Cited by
-
A DFT-based study of the hydrogen-bonding interactions between myricetin and ethanol/water.J Mol Model. 2019 Feb 14;25(3):67. doi: 10.1007/s00894-019-3940-8. J Mol Model. 2019. PMID: 30762117
-
A computational study on the reaction between fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH).J Mol Model. 2019 Mar 28;25(4):103. doi: 10.1007/s00894-019-3969-8. J Mol Model. 2019. PMID: 30923925
-
Promising Schiff bases in antiviral drug design and discovery.Med Chem Res. 2023;32(6):1063-1076. doi: 10.1007/s00044-023-03068-0. Epub 2023 May 10. Med Chem Res. 2023. PMID: 37305208 Free PMC article. Review.
-
ALA-Induced Flavonols Accumulation in Guard Cells Is Involved in Scavenging H2O2 and Inhibiting Stomatal Closure in Arabidopsis Cotyledons.Front Plant Sci. 2016 Nov 15;7:1713. doi: 10.3389/fpls.2016.01713. eCollection 2016. Front Plant Sci. 2016. PMID: 27895660 Free PMC article.
-
Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
