A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons
- PMID: 22196330
- PMCID: PMC3275419
- DOI: 10.1016/j.neuron.2011.12.002
A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons
Abstract
Neurotropic viruses that conditionally infect or replicate in molecularly defined neuronal subpopulations, and then spread transsynaptically, are powerful tools for mapping neural pathways. Genetically targetable retrograde transsynaptic tracer viruses are available to map the inputs to specific neuronal subpopulations, but an analogous tool for mapping synaptic outputs is not yet available. Here we describe a Cre recombinase-dependent, anterograde transneuronal tracer, based on the H129 strain of herpes simplex virus (HSV). Application of this virus to transgenic or knockin mice expressing Cre in peripheral neurons of the olfactory epithelium or the retina reveals widespread, polysynaptic labeling of higher-order neurons in the olfactory and visual systems, respectively. Polysynaptic pathways were also labeled from cerebellar Purkinje cells. In each system, the pattern of labeling was consistent with classical circuit-tracing studies, restricted to neurons, and anterograde specific. These data provide proof-of-principle for a conditional, nondiluting anterograde transsynaptic tracer for mapping synaptic outputs from genetically marked neuronal subpopulations.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

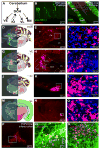

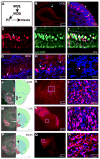


References
-
- Archin NM, van den Boom L, Perelygina L, Hilliard JM, Atherton SS. Delayed spread and reduction in virus titer after anterior chamber inoculation of a recombinant of HSV-1 expressing IL-16. Invest Ophthalmol Vis Sci. 2003;44:3066–3076. - PubMed
-
- Astic L, Saucier D, Coulon P, Lafay F, Flamand A. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res. 1993;619:146–156. - PubMed
-
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. - PubMed
-
- Bak IJ, Markham CH, Cook ML, Stevens JG. Intraaxonal transport of Herpes simplex virus in the rat central nervous system. Brain Res. 1977;136:415–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

