The functional organization of cutaneous low-threshold mechanosensory neurons
- PMID: 22196735
- PMCID: PMC3262167
- DOI: 10.1016/j.cell.2011.11.027
The functional organization of cutaneous low-threshold mechanosensory neurons
Abstract
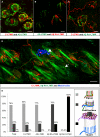
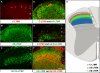
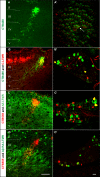
Innocuous touch of the skin is detected by distinct populations of neurons, the low-threshold mechanoreceptors (LTMRs), which are classified as Aβ-, Aδ-, and C-LTMRs. Here, we report genetic labeling of LTMR subtypes and visualization of their relative patterns of axonal endings in hairy skin and the spinal cord. We found that each of the three major hair follicle types of trunk hairy skin (guard, awl/auchene, and zigzag hairs) is innervated by a unique and invariant combination of LTMRs; thus, each hair follicle type is a functionally distinct mechanosensory end organ. Moreover, the central projections of Aβ-, Aδ-, and C-LTMRs that innervate the same or adjacent hair follicles form narrow LTMR columns in the dorsal horn. These findings support a model of mechanosensation in which the activities of Aβ-, Aδ-, and C-LTMRs are integrated within dorsal horn LTMR columns and processed into outputs that underlie the perception of myriad touch sensations.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. - PubMed
-
- Brown AG. Organization in the spinal cord: the anatomy and physiology of identified neurones. illustrated edn. Springer-Verlag; Berlin ; New York: 1981a. 1981.
-
- Brown AG. The spinocervical tract. Prog Neurobiol. 1981b;17:59–96. - PubMed
-
- Brown AG, Franz DN. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7:231–249. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS044094/NS/NINDS NIH HHS/United States
- R01 NS034814/NS/NINDS NIH HHS/United States
- R56 NS023725/NS/NINDS NIH HHS/United States
- N01 NS-7-2370/NS/NINDS NIH HHS/United States
- NS44094/NS/NINDS NIH HHS/United States
- MH084020/MH/NIMH NIH HHS/United States
- R01 NS052848/NS/NINDS NIH HHS/United States
- R37 NS034814/NS/NINDS NIH HHS/United States
- R01 NS023725/NS/NINDS NIH HHS/United States
- NS34814/NS/NINDS NIH HHS/United States
- NS052848/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- NS023725/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

