Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloë festucae in culture and endophyte-infected Lolium perenne
- PMID: 22196939
- PMCID: PMC3311397
- DOI: 10.1016/j.phytochem.2011.11.020
Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloë festucae in culture and endophyte-infected Lolium perenne
Abstract
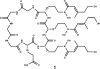
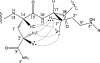
A number of genes encoding non-ribosomal peptide synthetases (NRPSs) have been identified in fungi of Epichloë/Neotyphodium species, endophytes of Pooid grasses, including sidN, putatively encoding a ferrichrome siderophore-synthesizing NRPS. Targeted gene replacement and complementation of sidN in Epichloë festucae has established that extracellular siderophore epichloënin A is the major product of the SidN enzyme complex (Johnson et al., 2007a). We report here high resolution mass spectrometric fragmentation experiments and NMR analysis of an isolated fraction establishing that epichloënin A is a siderophore of the ferrichrome family, comprising a cyclic sequence of four glycines, a glutamine and three N(δ)-trans-anhydromevalonyl-N(δ)-hydroxyornithine (AMHO) moieties. Epichloënin A is unusual among ferrichrome siderophores in comprising an octapeptide rather than hexapeptide sequence, and in incorporating a glutamine residue. During this investigation we have established that desferrichrome siderophores with pendant trans-AMHO groups can be distinguished from those with pendant cis-AMHO groups by the characteristic neutral loss of an hydroxyornithine moiety in the MS/MS spectrum. A minor component, epichloënin B, has been characterized as the triglycine variant by mass spectrometry. A peptide characterized by mass spectrometry as the putative deoxygenation product, epichloëamide has been detected together with ferriepichloënin A in guttation fluid from ryegrass (Lolium perenne) plants infected with wild-type E. festucae, but not in plants infected with the ΔsidN mutant strain, and also detected at trace levels in wild-type E. festucae fungal culture.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures






References
-
- Barnes C.L., Hosain M.B., Jalal M.A.F., Eng-Wilmot D.L., Grayson S.L., Benson B.A., Agarwal S.K., Mocherla R., van der Helm D. Ferrichrome conformation: ferrirubin, two crystal forms: C41H64FeN9O17·101/2H2O(I) and C41H64FeN9O17·CH3CN.H2O (II) Acta Crystallogr. C. 1985;41:341–347.
-
- Budzikiewicz, H., 2010. Microbial siderophores. Fortschr. Chem. Org. Naturst., vol. 92. Springer-Verlag, Vienna, pp. 1–75. - PubMed
-
- Bush L.P., Fannin F.F., Siegel M.R., Dahlman D.L., Burton H.R. Chemistry, occurrence and biological effects of saturated pyrrolizidine alkaloids associated with endophyte-grass interactions. Agric. Ecosyst. Environ. 1993;44:81–102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

