Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial
- PMID: 22196945
- PMCID: PMC3539769
- DOI: 10.1016/S0140-6736(11)61653-X
Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial
Abstract
Background: Nevirapine given once-daily for the first 6, 14, or 28 weeks of life to infants exposed to HIV-1 via breastfeeding reduces transmission through this route compared with single-dose nevirapine at birth or neonatally. We aimed to assess incremental safety and efficacy of extension of such prophylaxis to 6 months.
Methods: In our phase 3, randomised, double-blind, placebo-controlled HPTN 046 trial, we assessed the incremental benefit of extension of once-daily infant nevirapine from age 6 weeks to 6 months. We enrolled breastfeeding infants born to mothers with HIV-1 in four African countries within 7 days of birth. Following receipt of nevirapine from birth to 6 weeks, infants without HIV infection were randomly allocated (by use of a computer-generated permuted block algorithm with random block sizes and stratified by site and maternal antiretroviral treatment status) to receive extended nevirapine prophylaxis or placebo until 6 months or until breastfeeding cessation, whichever came first. The primary efficacy endpoint was HIV-1 infection in infants at 6 months and safety endpoints were adverse reactions in both groups. We used Kaplan-Meier analyses to compare differences in the primary outcome between groups. This study is registered with ClinicalTrials.gov, number NCT00074412.
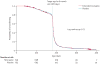
Findings: Between June 19, 2008, and March 12, 2010, we randomly allocated 1527 infants (762 nevirapine and 765 placebo); five of whom had HIV-1 infection at randomisation and were excluded from the primary analyses. In Kaplan-Meier analysis, 1·1% (95% CI 0·3-1·8) of infants who received extended nevirapine developed HIV-1 between 6 weeks and 6 months compared with 2·4% (1·3-3·6) of controls (difference 1·3%, 95% CI 0-2·6), equating to a 54% reduction in transmission (p=0·049). However, mortality (1·2% for nevirapine vs 1·1% for placebo; p=0·81) and combined HIV infection and mortality rates (2·3%vs 3·2%; p=0·27) did not differ between groups at 6 months. 125 (16%) of 758 infants given extended nevirapine and 116 (15%) of 761 controls had serious adverse events, but frequency of adverse events, serious adverse events, and deaths did not differ significantly between treatment groups.
Interpretation: Nevirapine prophylaxis can safely be used to provide protection from mother-to-child transmission of HIV-1 via breastfeeding for infants up to 6 months of age.
Funding: US National Institutes of Health.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Nevirapine prophylaxis during breastfeeding.Lancet. 2012 May 12;379(9828):1787-8; author reply 1788. doi: 10.1016/S0140-6736(12)60755-7. Lancet. 2012. PMID: 22579315 No abstract available.
References
-
- Coutsoudis A, Dabis F, Fawzi W, et al. and the Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. - PubMed
-
- Leroy V, Karon JM, Alioum A, et al. and the West Africa PMTCT Study Group. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–41. - PubMed
-
- Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–86. - PubMed
-
- WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–55. - PubMed
-
- Bedri A, Gudetta B, Isehak A, et al. and the Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

