Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia
- PMID: 22197325
- PMCID: PMC3253887
- DOI: 10.1016/j.cmet.2011.11.010
Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia
Abstract
The association of type 2 diabetes with elevated plasma triglyceride (TG) and very low-density lipoproteins (VLDL), and intrahepatic lipid accumulation represents a pathophysiological enigma and an unmet therapeutic challenge. Here, we uncover a link between insulin action through FoxO1, bile acid (BA) composition, and altered lipid homeostasis that brings new insight to this longstanding conundrum. FoxO1 ablation brings about two signature lipid abnormalities of diabetes and the metabolic syndrome, elevated liver and plasma TG. These changes are associated with deficiency of 12α-hydroxylated BAs and their synthetic enzyme, Cyp8b1, that hinders the TG-lowering effects of the BA receptor, Fxr. Accordingly, pharmacological activation of Fxr with GW4064 overcomes the BA imbalance, restoring hepatic and plasma TG levels of FoxO1-deficient mice to normal levels. We propose that generation of 12α-hydroxylated products of BA metabolism represents a signaling mechanism linking hepatic lipid abnormalities with type 2 diabetes, and a treatment target for this condition.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
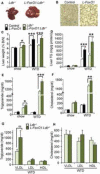
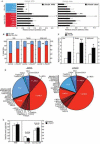
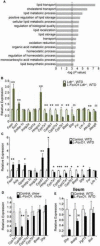

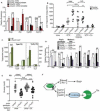
References
-
- Akiyoshi T, Uchida K, Takase H, Nomura Y, Takeuchi N. Cholesterol gallstones in alloxan-diabetic mice. J Lipid Res. 1986;27:915–924. - PubMed
-
- Angelin B, Einarsson K, Hellstrom K, Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978;19:1017–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

