Diversity, biogenesis and function of microbial amyloids
- PMID: 22197327
- PMCID: PMC3278576
- DOI: 10.1016/j.tim.2011.11.005
Diversity, biogenesis and function of microbial amyloids
Abstract
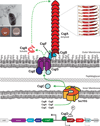
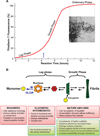
Amyloid is a distinct β-sheet-rich fold that many proteins can acquire. Frequently associated with neurodegenerative diseases in humans, including Alzheimer's, Parkinson's and Huntington's diseases, amyloids are traditionally considered the product of protein misfolding. However, the amyloid fold is now recognized as a ubiquitous part of normal cellular biology. Functional amyloids have been identified in nearly all facets of cellular life, with microbial functional amyloids leading the way. Unlike disease-associated amyloids, functional amyloids are assembled by dedicated, directed pathways and ultimately perform a physiological function that benefits the organism. The evolved amyloid assembly and disassembly pathways of microbes have provided novel insights into how cells have harnessed the amyloid assembly process for productive means. An understanding of functional amyloid biogenesis promises to provide a fresh perspective on the molecular events that underlie disease-associated amyloidogenesis. Here, we review functional microbial amyloids with an emphasis on curli fibers and their role in promoting biofilm formation and other community behaviors.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Dobson CM. Structural biology: prying into prions. Nature. 2005;435:747–749. - PubMed
-
- Collinson SK, Parker JM, Hodges RS, Kay WW. Structural predictions of AgfA, the insoluble fimbrial subunit of Salmonella thin aggregative fimbriae. J Mol Biol. 1999;290:741–756. - PubMed
-
- Nordstedt C, Naslund J, Tjernberg LO, Karlstrom AR, Thyberg J, Terenius L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. J Biol Chem. 1994;269:30773–30776. - PubMed
-
- Sitaras C, Naghavi M, Herrington MB. Sodium dodecyl sulfate-agarose gel electrophoresis for the detection and isolation of amyloid curli fibers. Anal Biochem. 2011;408:328–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

