Ligand binding and membrane insertion compete with oligomerization of the BclXL apoptotic repressor
- PMID: 22197371
- PMCID: PMC3268818
- DOI: 10.1016/j.jmb.2011.12.015
Ligand binding and membrane insertion compete with oligomerization of the BclXL apoptotic repressor
Abstract
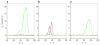
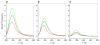
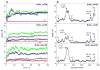
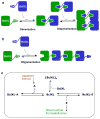
B-cell lymphoma extra large (BclXL) apoptotic repressor plays a central role in determining the fate of cells to live or die during physiological processes such as embryonic development and tissue homeostasis. Herein, using a myriad of biophysical techniques, we provide evidence that ligand binding and membrane insertion compete with oligomerization of BclXL in solution. Of particular importance is the observation that such oligomerization is driven by the intermolecular binding of its C-terminal transmembrane (TM) domain to the canonical hydrophobic groove in a domain-swapped trans fashion, whereby the TM domain of one monomer occupies the canonical hydrophobic groove within the other monomer and vice versa. Binding of BH3 ligands to the canonical hydrophobic groove displaces the TM domain in a competitive manner, allowing BclXL to dissociate into monomers upon hetero-association. Remarkably, spontaneous insertion of BclXL into DMPC/DHPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine/1,2-dihexanoyl-sn-glycero-3-phosphocholine) bicelles results in a dramatic conformational change such that it can no longer recognize the BH3 ligands in what has come to be known as the "hit-and-run" mechanism. Collectively, our data suggest that oligomerization of a key apoptotic repressor serves as an allosteric switch that fine-tunes its ligand binding and membrane insertion pertinent to the regulation of apoptotic machinery.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures






References
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. - PubMed
-
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406. - PubMed
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
-
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

