Mechanical derivation of functional myotubes from adipose-derived stem cells
- PMID: 22197570
- PMCID: PMC3261363
- DOI: 10.1016/j.biomaterials.2011.12.004
Mechanical derivation of functional myotubes from adipose-derived stem cells
Abstract
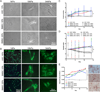
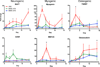
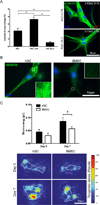
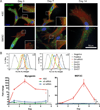
Though reduced serum or myoblast co-culture alone can differentiate adipose-derived stem cells (ASCs) into mesenchymal lineages, efficiency is usually not sufficient to restore function in vivo. Often when injected into fibrotic muscle, their differentiation may be misdirected by the now stiffened tissue. Here ASCs are shown to not just simply reflect the qualitative stiffness sensitivity of bone marrow-derived stem cells (BMSCs) but to exceed BMSC myogenic capacity, expressing the appropriate temporal sequence of muscle transcriptional regulators on muscle-mimicking extracellular matrix in a tension and focal adhesion-dependent manner. ASCs formed multi-nucleated myotubes with a continuous cytoskeleton that was not due to misdirected cell division; microtubule depolymerization severed myotubes, but after washout, ASCs refused at a rate similar to pre-treated values. BMSCs never underwent stiffness-mediated fusion. ASC-derived myotubes, when replated onto non-permissive stiff matrix, maintained their fused state. Together these data imply enhanced mechanosensitivity for ASCs, making them a better therapeutic cell source for fibrotic muscle.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures

References
-
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Davies JE, Rose C, Sarkar S, Rubinsztein DC. Cystamine suppresses polyalanine toxicity in a mouse model of oculopharyngeal muscular dystrophy. Sci Transl Med. 2010;2 34ra40. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

