Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse
- PMID: 22197678
- PMCID: PMC3580389
- DOI: 10.1016/j.bbr.2011.12.019
Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse
Abstract
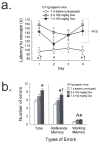
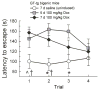
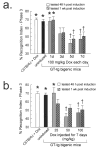
HIV-Tat protein has been implicated in the pathogenesis of HIV-1 neurological complications (i.e., neuroAIDS), but direct demonstrations of the effects of Tat on behavior are limited. GT-tg mice with a doxycycline (Dox)-inducible and brain-selective tat gene coding for Tat protein were used to test the hypothesis that the activity of Tat in brain is sufficient to impair learning and memory processes. Western blot analysis of GT-tg mouse brains demonstrated an increase in Tat antibody labeling that seemed to be dependent on the dose and duration of Dox pretreatment. Dox-treated GT-tg mice tested in the Barnes maze demonstrated longer latencies to find an escape hole and displayed deficits in probe trial performance versus uninduced GT-tg littermates, suggesting Tat-induced impairments of spatial learning and memory. Reversal learning was also impaired in Tat-induced mice. Tat-induced mice additionally demonstrated long-lasting (up to one month) deficiencies in novel object recognition learning and memory performance. Furthermore, novel object recognition impairment was dependent on the dose and duration of Dox exposure, suggesting that Tat exposure progressively mediated deficits. These experiments provide evidence that Tat protein expression is sufficient to mediate cognitive abnormalities seen in HIV-infected individuals. Moreover, the genetically engineered GT-tg mouse may be useful for improving our understanding of the neurological underpinnings of neuroAIDS-related behaviors.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


References
-
- Frankel AD, Young JA. HIV-1 fifteen proteins and an RNA. Ann Rev Biochem. 1998;67:1–25. - PubMed
-
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. - PubMed
-
- Johnston JB, Zhang K, Silva C, Shalinsky DR, Conant K, Ni W, Corbett D, Yong VW, Power C. HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol. 2001;49:230–241. - PubMed
-
- Glowa JR, Panlilio LV, Brenneman DE, Gozes I, Fridkin M, Hill JM. Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 1992;570:49–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

