Serotonergic and dopaminergic distinctions in the behavioral pharmacology of (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD)
- PMID: 22197710
- PMCID: PMC3272148
- DOI: 10.1016/j.pbb.2011.12.002
Serotonergic and dopaminergic distinctions in the behavioral pharmacology of (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD)
Abstract
Rationale: After decades of social stigma, hallucinogens have reappeared in the clinical literature demonstrating unique benefits in medicine. The precise behavioral pharmacology of these compounds remains unclear, however.
Objectives: Two commonly studied hallucinogens, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD), were investigated both in vivo and in vitro to determine the pharmacology of their behavioral effects in an animal model.
Method: Rabbits were administered DOI or LSD and observed for head bob behavior after chronic drug treatment or after pretreatment with antagonist ligands. The receptor binding characteristics of DOI and LSD were studied in vitro in frontocortical homogenates from naïve rabbits or ex vivo in animals receiving an acute drug injection.
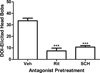
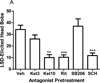
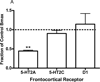
Results: Both DOI- and LSD-elicited head bobs required serotonin(2A) (5-HT(2A)) and dopamine(1) (D(1)) receptor activation. Serotonin(2B/2C) receptors were not implicated in these behaviors. In vitro studies demonstrated that LSD and the 5-HT(2A/2C) receptor antagonist, ritanserin, bound frontocortical 5-HT(2A) receptors in a pseudo-irreversible manner. In contrast, DOI and the 5-HT(2A/2C) receptor antagonist, ketanserin, bound reversibly. These binding properties were reflected in ex vivo binding studies. The two hallucinogens also differed in that LSD showed modest D(1) receptor binding affinity whereas DOI had negligible binding affinity at this receptor.
Conclusion: Although DOI and LSD differed in their receptor binding properties, activation of 5-HT(2A) and D(1) receptors was a common mechanism for eliciting head bob behavior. These findings implicate these two receptors in the mechanism of action of hallucinogens.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
For all authors there are no conflicts of interested involving these data.
Figures






References
-
- Aloyo VJ, Dave KD, Rahman T, Harvey JA. Selective and divergent regulation of cortical 5-HT(2A) receptors in rabbit. J Pharmacol Exp Ther. 2001;299(3):1066–1072. - PubMed
-
- Aloyo VJ, Harvey JA. Antagonist binding at 5-HT(2A) and 5-HT(2C) receptors in the rabbit: high correlation with the profile for the human receptors. Eur J Pharmacol. 2000;406(2):163–169. - PubMed
-
- Burris KD, Sanders-Bush E. Unsurmountable antagonism of brain 5-hydroxytryptamine2 receptors by (+)-lysergic acid diethylamide and bromo-lysergic acid diethylamide. Mol Pharmacol. 1992;42(5):826–830. - PubMed
-
- Burt DR, Creese I, Snyder SH. Binding interactions of lysergic acid diethylamide and related agents with dopamine receptors in the brain. Mol Pharmacol. 1976;12(4):631–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

