NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley
- PMID: 22197894
- PMCID: PMC3295391
- DOI: 10.1093/jxb/err400
NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley
Abstract
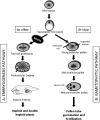
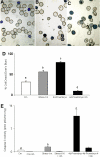
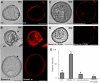
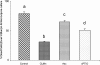
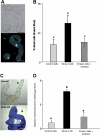
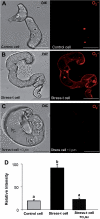
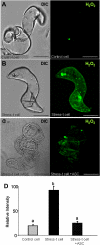
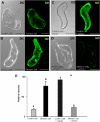
Under specific stress treatments (cold, starvation), in vitro microspores can be induced to deviate from their gametophytic development and switch to embryogenesis, forming haploid embryos and homozygous breeding lines in a short period of time. The inductive stress produces reactive oxygen species (ROS) and nitric oxide (NO), signalling molecules mediating cellular responses, and cell death, modifying the embryogenic microspore response and therefore, the efficiency of the process. This work analysed cell death, caspase 3-like activity, and ROS and NO production (using fluorescence probes and confocal analysis) after inductive stress in barley microspore cultures and embryogenic suspension cultures, as an in vitro system which permitted easy handling for comparison. There was an increase in caspase 3-like activity and cell death after stress treatment in microspore and suspension cultures, while ROS increased in non-induced microspores and suspension cultures. Treatments of the cultures with a caspase 3 inhibitor, DEVD-CHO, significantly reduced the cell death percentages. Stress-treated embryogenic suspension cultures exhibited high NO signals and cell death, while treatment with S-nitrosoglutathione (NO donor) in control suspension cultures resulted in even higher cell death. In contrast, in microspore cultures, NO production was detected after stress, and, in the case of 4-day microspore cultures, in embryogenic microspores accompanying the initiation of cell divisions. Subsequent treatments of stress-treated microspore cultures with ROS and NO scavengers resulted in a decreasing cell death during the early stages, but later they produced a delay in embryo development as well as a decrease in the percentage of embryogenesis in microspores. Results showed that the ROS increase was involved in the stress-induced programmed cell death occurring at early stages in both non-induced microspores and embryogenic suspension cultures; whereas NO played a dual role after stress in the two in vitro systems, one involved in programmed cell death in embryogenic suspension cultures and the other in the initiation of cell division leading to embryogenesis in reprogrammed microspores.
Figures







References
-
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. - PubMed
-
- Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F. An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. Journal of Biological Chemistry. 2006;281:23579–23588. - PubMed
-
- Balestrazzi A, Agoni V, Tava A, Avato P, Biazzi E, Raimondi E, Macovei A, Carbonera D. Cell death induction and nitric oxide biosynthesis in white poplar (Populus alba) suspension cultures exposed to alfalfa saponins. Physiologia Plantarum. 2011;141:227–238. - PubMed
-
- Beligni MV, Lamattina L. Nitric oxide in plants: the history is just beginning. Plant, Cell and Environment. 2001;24:267–278.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

