Mice completely lacking immunoproteasomes show major changes in antigen presentation
- PMID: 22197977
- PMCID: PMC3262888
- DOI: 10.1038/ni.2203
Mice completely lacking immunoproteasomes show major changes in antigen presentation
Abstract
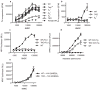
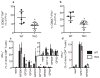
The importance of immunoproteasomes to antigen presentation has been unclear because animals totally lacking immunoproteasomes had not been available. Having now developed mice lacking the three immunoproteasome catalytic subunits, we found that the dendritic cells of these mice had defects in presenting several major histocompatibility complex (MHC) class I epitopes. During viral infection in vivo, the presentation of a majority of MHC class I epitopes was markedly reduced in immunoproteasome-deficient animals compared with wild-type animals, whereas presentation of MHC class II peptides was unaffected. According to mass spectrometry, the repertoire of MHC class I-presented peptides was ∼50% different from that in wild-type mice, and these differences were sufficient to stimulate robust transplant rejection of wild-type cells in mutant mice. These results indicated that immunoproteasomes were more important in antigen presentation than previously thought.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures




Comment in
-
Immuno-waste exposure and further management.Nat Immunol. 2012 Jan 19;13(2):109-11. doi: 10.1038/ni.2204. Nat Immunol. 2012. PMID: 22261957 No abstract available.
References
-
- Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
-
- Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. - PubMed
-
- Tanaka K. Role of proteasomes modified by interferon-gamma in antigen processing. J Leukoc Biol. 1994;56:571–575. - PubMed
-
- Gaczynska M, Rock KL, Goldberg AL. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. - PubMed
-
- Driscoll J, Brown MG, Finley D, Monaco JJ. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993;365:262–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

