Phloem ultrastructure and pressure flow: Sieve-Element-Occlusion-Related agglomerations do not affect translocation
- PMID: 22198148
- PMCID: PMC3269875
- DOI: 10.1105/tpc.111.093179
Phloem ultrastructure and pressure flow: Sieve-Element-Occlusion-Related agglomerations do not affect translocation
Abstract
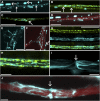
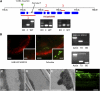
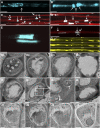
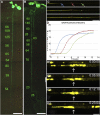
Since the first ultrastructural investigations of sieve tubes in the early 1960s, their structure has been a matter of debate. Because sieve tube structure defines frictional interactions in the tube system, the presence of P protein obstructions shown in many transmission electron micrographs led to a discussion about the mode of phloem transport. At present, it is generally agreed that P protein agglomerations are preparation artifacts due to injury, the lumen of sieve tubes is free of obstructions, and phloem flow is driven by an osmotically generated pressure differential according to Münch's classical hypothesis. Here, we show that the phloem contains a distinctive network of protein filaments. Stable transgenic lines expressing Arabidopsis thaliana Sieve-Element-Occlusion-Related1 (SEOR1)-yellow fluorescent protein fusions show that At SEOR1 meshworks at the margins and clots in the lumen are a general feature of living sieve tubes. Live imaging of phloem flow and flow velocity measurements in individual tubes indicate that At SEOR1 agglomerations do not markedly affect or alter flow. A transmission electron microscopy preparation protocol has been generated showing sieve tube ultrastructure of unprecedented quality. A reconstruction of sieve tube ultrastructure served as basis for tube resistance calculations. The impact of agglomerations on phloem flow is discussed.
Figures





Similar articles
-
SEORious business: structural proteins in sieve tubes and their involvement in sieve element occlusion.J Exp Bot. 2014 Apr;65(7):1879-93. doi: 10.1093/jxb/eru071. Epub 2014 Mar 3. J Exp Bot. 2014. PMID: 24591057 Review.
-
The structure of the phloem--still more questions than answers.Plant J. 2012 Apr;70(1):147-56. doi: 10.1111/j.1365-313X.2012.04931.x. Plant J. 2012. PMID: 22449049 Review.
-
Intra-annual dynamics of phloem formation and ultrastructural changes in sieve tubes in Fagus sylvatica.Tree Physiol. 2019 Feb 1;39(2):262-274. doi: 10.1093/treephys/tpy102. Tree Physiol. 2019. PMID: 30239917
-
Identification of phloem-specific proteinaSEOus structure heterogeneity in sieve element of Populus trichocarpa.BMC Plant Biol. 2025 Apr 10;25(1):456. doi: 10.1186/s12870-025-06439-4. BMC Plant Biol. 2025. PMID: 40211162 Free PMC article.
-
Sieve element occlusion (SEO) genes encode structural phloem proteins involved in wound sealing of the phloem.Proc Natl Acad Sci U S A. 2012 Jul 10;109(28):E1980-9. doi: 10.1073/pnas.1202999109. Epub 2012 Jun 25. Proc Natl Acad Sci U S A. 2012. PMID: 22733783 Free PMC article.
Cited by
-
Characterization of Local and Systemic Impact of Whitefly (Bemisia tabaci) Feeding and Whitefly-Transmitted Tomato Mottle Virus Infection on Tomato Leaves by Comprehensive Proteomics.Int J Mol Sci. 2020 Sep 30;21(19):7241. doi: 10.3390/ijms21197241. Int J Mol Sci. 2020. PMID: 33008056 Free PMC article.
-
Comparative Proteomics Analysis of Phloem Exudates Collected during the Induction of Systemic Acquired Resistance.Plant Physiol. 2016 Jun;171(2):1495-510. doi: 10.1104/pp.16.00269. Epub 2016 Apr 19. Plant Physiol. 2016. PMID: 27208255 Free PMC article.
-
Identification of novel regulators required for early development of vein pattern in the cotyledons by single-cell RNA-sequencing.Plant J. 2022 Apr;110(1):7-22. doi: 10.1111/tpj.15719. Epub 2022 Mar 16. Plant J. 2022. PMID: 35218590 Free PMC article.
-
MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs.Nature. 2018 Jan 3;553(7686):82-85. doi: 10.1038/nature25027. Nature. 2018. PMID: 29300014
-
Plant MicroRNAs in Cross-Kingdom Regulation of Gene Expression.Int J Mol Sci. 2018 Jul 10;19(7):2007. doi: 10.3390/ijms19072007. Int J Mol Sci. 2018. PMID: 29996470 Free PMC article. Review.
References
-
- Bánó M., Marek J. (2006). How thick is the layer of thermal volume surrounding the protein? Biophys. Chem. 120: 44–54 - PubMed
-
- Becker D., Kemper E., Schell J., Masterson R. (1992). New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol. 20: 1195–1197 - PubMed
-
- Bozzola J.J., Russell L.D. (1999). Electron Microscopy: Principles and Techniques for Biologists. (Sudbury, MA: Jones & Bartlett Learning)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

