In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis
- PMID: 22198198
- PMCID: PMC4380186
- DOI: 10.1007/s10456-011-9245-x
In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis
Abstract
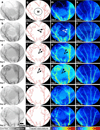
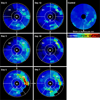
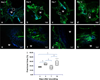
Laser speckle contrast imaging (LSCI) is a high-resolution and high contrast optical imaging technique often used to characterize hemodynamic changes in short-term physiological experiments. In this study, we demonstrate the utility of LSCI for characterizing microvascular remodeling and hemodynamic changes during wound healing angiogenesis in vivo. A 2 mm diameter hole was made in the mouse ear and the periphery of the wound imaged in vivo using LSCI over 12 days. We were able to visualize and quantify the vascular and perfusion changes that accompanied wound healing in the microenvironment proximal to the wound, and validated these changes with histology. We found that consistent with the stages of wound healing, microvessel density increased during the initial inflammatory phase (i.e., day 0-3), stayed elevated through the tissue formation phase (i.e., until day 7) and returned to baseline during the tissue remodeling phase (i.e., by day 12). Concomitant "wide area mapping" of blood flow revealed that tissue perfusion in the wound periphery initially decreased, gradually increased from day 3-7, and subsided as healing completed. Interestingly, some regions exhibited a reestablishment of tissue perfusion approximately 6 days earlier than the ~18 days usually reported for the long term remodeling phase. The results from this study demonstrate that LSCI is an ideal platform for elucidating in vivo changes in microvascular hemodynamics and angiogenesis, and has the potential to offer invaluable insights in a range of disease models involving abnormal hemodynamics, such as diabetes and tumors.
Figures


References
-
- Singer AJ, Clark RAF. Cutaneous wound healing. New Eng J Med. 1999;341:738–746. - PubMed
-
- Kiyama T, Naito M, Shitama H, Shinoda T, Maeyama A. Comparison of skin blood flow between mini- and standard-incision approaches during total hip arthroplasty. J Arthroplast. 2008;23:1045–1049. - PubMed
-
- Ngo BT, Hayes KD, DiMiao DJ, Srinivasan SK, Huerter CJ, et al. Manifestations of cutaneous diabetic microangiopathy. Am J Clin Dermatol. 2005;6:225–237. - PubMed
-
- Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34:593–600. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

