Manganese superoxide dismutase, MnSOD and its mimics
- PMID: 22198225
- PMCID: PMC3304004
- DOI: 10.1016/j.bbadis.2011.12.002
Manganese superoxide dismutase, MnSOD and its mimics
Abstract
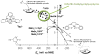
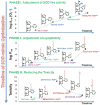
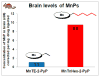
Increased understanding of the role of mitochondria under physiological and pathological conditions parallels increased exploration of synthetic and natural compounds able to mimic MnSOD - endogenous mitochondrial antioxidant defense essential for the existence of virtually all aerobic organisms from bacteria to humans. This review describes most successful mitochondrially-targeted redox-active compounds, Mn porphyrins and MitoQ(10) in detail, and briefly addresses several other compounds that are either catalysts of O(2)(-) dismutation, or its non-catalytic scavengers, and that reportedly attenuate mitochondrial dysfunction. While not a true catalyst (SOD mimic) of O(2)(-) dismutation, MitoQ(10) oxidizes O(2)(-) to O(2) with a high rate constant. In vivo it is readily reduced to quinol, MitoQH(2), which in turn reduces ONOO(-) to NO(2), producing semiquinone radical that subsequently dismutes to MitoQ(10) and MitoQH(2), completing the "catalytic" cycle. In MitoQ(10), the redox-active unit was coupled via 10-carbon atom alkyl chain to monocationic triphenylphosphonium ion in order to reach the mitochondria. Mn porphyrin-based SOD mimics, however, were designed so that their multiple cationic charge and alkyl chains determine both their remarkable SOD potency and carry them into the mitochondria. Several animal efficacy studies such as skin carcinogenesis and UVB-mediated mtDNA damage, and subcellular distribution studies of Saccharomyces cerevisiae and mouse heart provided unambiguous evidence that Mn porphyrins mimic the site and action of MnSOD, which in turn contributes to their efficacy in numerous in vitro and in vivo models of oxidative stress. Within a class of Mn porphyrins, lipophilic analogs are particularly effective for treating central nervous system injuries where mitochondria play key role. This article is part of a Special Issue entitled: Antioxidants and Antioxidant Treatment in Disease.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

 - normal cell;
- normal cell;
 - transformed cell;
- transformed cell;
 - cancer cell.
- cancer cell.

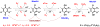








Similar articles
-
An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways.Redox Biol. 2015 Aug;5:43-65. doi: 10.1016/j.redox.2015.01.017. Epub 2015 Feb 7. Redox Biol. 2015. PMID: 25827425 Free PMC article. Review.
-
Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics.Free Radic Biol Med. 2011 Sep 1;51(5):1035-53. doi: 10.1016/j.freeradbiomed.2011.04.046. Epub 2011 May 6. Free Radic Biol Med. 2011. PMID: 21616142 Free PMC article. Review.
-
Methoxy-derivatization of alkyl chains increases the in vivo efficacy of cationic Mn porphyrins. Synthesis, characterization, SOD-like activity, and SOD-deficient E. coli study of meta Mn(III) N-methoxyalkylpyridylporphyrins.Dalton Trans. 2011 Apr 28;40(16):4111-21. doi: 10.1039/c0dt01321h. Epub 2011 Mar 8. Dalton Trans. 2011. PMID: 21384047 Free PMC article.
-
A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity.Free Radic Biol Med. 2012 May 1;52(9):1828-34. doi: 10.1016/j.freeradbiomed.2012.02.006. Epub 2012 Feb 13. Free Radic Biol Med. 2012. PMID: 22336516 Free PMC article.
-
A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics.Free Radic Biol Med. 2015 Sep;86:308-21. doi: 10.1016/j.freeradbiomed.2015.05.018. Epub 2015 May 28. Free Radic Biol Med. 2015. PMID: 26026699 Free PMC article.
Cited by
-
Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications.Antioxid Redox Signal. 2015 Mar 10;22(8):686-729. doi: 10.1089/ars.2014.5952. Antioxid Redox Signal. 2015. PMID: 25546574 Free PMC article. Review.
-
Comprehensive pharmacokinetic studies and oral bioavailability of two Mn porphyrin-based SOD mimics, MnTE-2-PyP5+ and MnTnHex-2-PyP5+.Free Radic Biol Med. 2013 May;58:73-80. doi: 10.1016/j.freeradbiomed.2013.01.006. Epub 2013 Jan 15. Free Radic Biol Med. 2013. PMID: 23328731 Free PMC article.
-
Preliminary Study to Assess the Impact of Dietary Rutin on Growth, Antioxidant Capacity, and Intestinal Health of Yellow Catfish, Pelteobagrus fulvidraco.Animals (Basel). 2023 Oct 31;13(21):3386. doi: 10.3390/ani13213386. Animals (Basel). 2023. PMID: 37958140 Free PMC article.
-
The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets.Vet Sci. 2023 Nov 10;10(11):650. doi: 10.3390/vetsci10110650. Vet Sci. 2023. PMID: 37999473 Free PMC article.
-
Geometrical variations of two manganese(II) complexes with closely related quinoline-based tripodal ligands.Acta Crystallogr E Crystallogr Commun. 2021 Sep 28;77(Pt 10):982-988. doi: 10.1107/S2056989021009786. eCollection 2021 Oct 1. Acta Crystallogr E Crystallogr Commun. 2021. PMID: 34667623 Free PMC article.
References
-
- Christianson DW. Structural chemistry and biology of manganese metalloenzymes. Prog Biophys Mol Biol. 1997;67:217–252. - PubMed
-
- Li JJ, Oberley LW, St Clair DK, Ridnour LA, Oberley TD. Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene. 1995;10:1989–2000. - PubMed
-
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

