Allostery and cooperativity in Escherichia coli aspartate transcarbamoylase
- PMID: 22198283
- PMCID: PMC3393099
- DOI: 10.1016/j.abb.2011.10.024
Allostery and cooperativity in Escherichia coli aspartate transcarbamoylase
Abstract
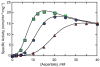
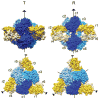
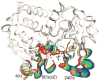
The allosteric enzyme aspartate transcarbamoylase (ATCase) from Escherichia coli has been the subject of investigations for approximately 50 years. This enzyme controls the rate of pyrimidine nucleotide biosynthesis by feedback inhibition, and helps to balance the pyrimidine and purine pools by competitive allosteric activation by ATP. The catalytic and regulatory components of the dodecameric enzyme can be separated and studied independently. Many of the properties of the enzyme follow the Monod, Wyman Changeux model of allosteric control thus E. coli ATCase has become the textbook example. This review will highlight kinetic, biophysical, and structural studies which have provided a molecular level understanding of how the allosteric nature of this enzyme regulates pyrimidine nucleotide biosynthesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
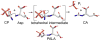





References
-
- Yates RA, Pardee AB. J Biol Chem. 1956;221:757–770. - PubMed
-
- Jones ME, Spector L, Lipmann F. J Am Chem Soc. 1955;77:819–820.
-
- Lowenstein JM, Cohen PP. J Biol Chem. 1956;235:57–78. - PubMed
-
- Reichard P, Hanshoff G. Acta Chem Scand. 1956;10:548–560.
-
- Shepherdson M, Pardee AB. J Biol Chem. 1960;235:3233–3237.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

