Pathophysiology of the cardiac late Na current and its potential as a drug target
- PMID: 22198344
- PMCID: PMC3816394
- DOI: 10.1016/j.yjmcc.2011.12.003
Pathophysiology of the cardiac late Na current and its potential as a drug target
Abstract
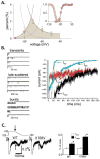
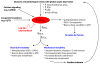
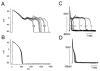
A pathological increase in the late component of the cardiac Na(+) current, I(NaL), has been linked to disease manifestation in inherited and acquired cardiac diseases including the long QT variant 3 (LQT3) syndrome and heart failure. Disruption in I(NaL) leads to action potential prolongation, disruption of normal cellular repolarization, development of arrhythmia triggers, and propensity to ventricular arrhythmia. Attempts to treat arrhythmogenic sequelae from inherited and acquired syndromes pharmacologically with common Na(+) channel blockers (e.g. flecainide, lidocaine, and amiodarone) have been largely unsuccessful. This is due to drug toxicity and the failure of most current drugs to discriminate between the peak current component, chiefly responsible for single cell excitability and propagation in coupled tissue, and the late component (I(NaL)) of the Na(+) current. Although small in magnitude as compared to the peak Na(+) current (~1-3%), I(NaL) alters action potential properties and increases Na(+) loading in cardiac cells. With the increasing recognition that multiple cardiac pathological conditions share phenotypic manifestations of I(NaL) upregulation, there has been renewed interest in specific pharmacological inhibition of I(Na). The novel antianginal agent ranolazine, which shows a marked selectivity for late versus peak Na(+) current, may represent a novel drug archetype for targeted reduction of I(NaL). This article aims to review common pathophysiological mechanisms leading to enhanced I(NaL) in LQT3 and heart failure as prototypical disease conditions. Also reviewed are promising therapeutic strategies tailored to alter the molecular mechanisms underlying I(Na) mediated arrhythmia triggers.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
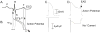
References
-
- Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol. 1979;236(4):H561–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

