Like a bolt from the blue: phthalocyanines in biomedical optics
- PMID: 22198535
- PMCID: PMC6269082
- DOI: 10.3390/molecules17010098
Like a bolt from the blue: phthalocyanines in biomedical optics
Abstract
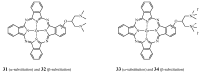
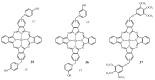
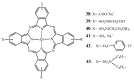
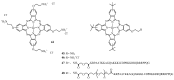
The purpose of this review is to compile preclinical and clinical results on phthalocyanines (Pcs) as photosensitizers (PS) for Photodynamic Therapy (PDT) and contrast agents for fluorescence imaging. Indeed, Pcs are excellent candidates in these fields due to their strong absorbance in the NIR region and high chemical and photo-stability. In particular, this is mostly relevant for their in vivo activation in deeper tissular regions. However, most Pcs present two major limitations, i.e., a strong tendency to aggregate and a low water-solubility. In order to overcome these issues, both chemical tuning and pharmaceutical formulation combined with tumor targeting strategies were applied. These aspects will be developed in this review for the most extensively studied Pcs during the last 25 years, i.e., aluminium-, zinc- and silicon-based Pcs.
Figures
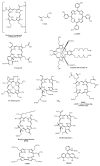
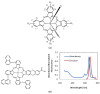
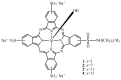
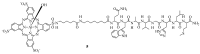
References
-
- McKeown N.B. The synthesis of symmetrical phthalocyanines. In: Kadish K.M., Smith K.M., Guilard R., editors. The Porphyrin Handbook. Academic Press; San Diego, CA, USA: 2003. pp. 61–124.
-
- Thomas A.L. Phthalocyanine Research and Applications. CRC Press; Baton Rouge, FL, USA: 1990. p. 2.
-
- McKeown N.B. An introduction to the phthalocyanines. In: McKeown N.B., editor. Phthalocyanine Materials: Synthesis, Structure and Function. Cambridge University Press; Cambridge, UK: 1998. pp. 1–10.
-
- Geng Y.Y., Gu D.H., Wu Y.Q., Gan F.X. High speed recording property of phthalocyanine thin film for compact disc recordable. Proc. SPIE Int. Soc. Opt. Eng. 2003;63:63–66.
-
- Petritsch K., Friend R.H., Lux A., Rozenberg G., Moratti S.C., Holmes A.B. Liquid crystalline phthalocyanines in organic solar cells. Synth. Met. 1999;102:1776–1777.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

