Examination of mesenchymal stem cell-mediated RNAi transfer to Huntington's disease affected neuronal cells for reduction of huntingtin
- PMID: 22198539
- PMCID: PMC3784251
- DOI: 10.1016/j.mcn.2011.12.001
Examination of mesenchymal stem cell-mediated RNAi transfer to Huntington's disease affected neuronal cells for reduction of huntingtin
Abstract
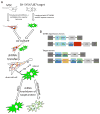
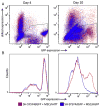
Huntington's disease (HD) is a fatal, autosomal dominant neurodegenerative disorder caused by an expanded trinucleotide (CAG) repeat in exon 1 of the huntingtin gene (Htt). This expansion creates a toxic polyglutamine tract in the huntingtin protein (HTT). Currently, there is no treatment for either the progression or prevention of the disease. RNA interference (RNAi) technology has shown promise in transgenic mouse models of HD by reducing expression of mutant HTT and slowing disease progression. The advancement of RNAi therapies to human clinical trials is hampered by problems delivering RNAi to affected neurons in a robust and sustainable manner. Mesenchymal stem cells (MSC) have demonstrated a strong safety profile in both completed and numerous ongoing clinical trials. MSC exhibit a number of innate therapeutic effects, such as immune system modulation, homing to injury, and cytokine release into damaged microenvironments. The ability of MSC to transfer larger molecules and even organelles suggested their potential usefulness as delivery vehicles for therapeutic RNA inhibition. In a series of model systems we have found evidence that MSC can transfer RNAi targeting both reporter genes and mutant huntingtin in neural cell lines. MSC expressing shRNA antisense to GFP were found to decrease expression of GFP in SH-SY5Y cells after co-culture when assayed by flow cytometry. Additionally MSC expressing shRNA antisense to HTT were able to decrease levels of mutant HTT expressed in both U87 and SH-SY5Y target cells when assayed by Western blot and densitometry. These results are encouraging for expanding the therapeutic abilities of both RNAi and MSC for future treatments of Huntington's disease.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



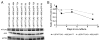

References
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

