Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex
- PMID: 22198573
- PMCID: PMC3276695
- DOI: 10.1016/j.nbd.2011.12.028
Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex
Abstract
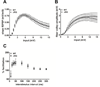
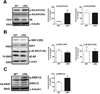
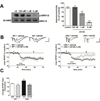
Tuberous sclerosis complex (TSC) and fragile X syndrome (FXS) are caused by mutations in negative regulators of translation. FXS model mice exhibit enhanced metabotropic glutamate receptor-dependent long-term depression (mGluR-LTD). Therefore, we hypothesized that a mouse model of TSC, ΔRG transgenic mice, also would exhibit enhanced mGluR-LTD. We measured the impact of TSC2-GAP mutations on the mTORC1 and ERK signaling pathways and protein synthesis-dependent hippocampal synaptic plasticity in ΔRG transgenic mice. These mice express a dominant/negative TSC2 that binds to TSC1, but has a deletion and substitution mutation in its GAP-domain, resulting in inactivation of the complex. Consistent with previous studies of several other lines of TSC model mice, we observed elevated S6 phosphorylation in the brains of ΔRG mice, suggesting upregulated translation. Surprisingly, mGluR-LTD was not enhanced, but rather was impaired in the ΔRG transgenic mice, indicating that TSC and FXS have divergent synaptic plasticity phenotypes. Similar to patients with TSC, the ΔRG transgenic mice exhibit elevated ERK signaling. Moreover, the mGluR-LTD impairment displayed by the ΔRG transgenic mice was rescued with the MEK-ERK inhibitor U0126. Our results suggest that the mGluR-LTD impairment observed in ΔRG mice involves aberrant TSC1/2-ERK signaling.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Boer K, et al. Cellular localization of metabotropic glutamate receptors in cortical tubers and subependymal giant cell tumorFs of tuberous sclerosis complex. Neuroscience. 2008;156:203–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

