Innate immune response to Salmonella typhimurium, a model enteric pathogen
- PMID: 22198618
- PMCID: PMC3370950
- DOI: 10.4161/gmic.19141
Innate immune response to Salmonella typhimurium, a model enteric pathogen
Abstract
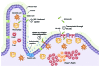
The innate immune system provides the first line of defense against invading microorganisms by inducing a variety of inflammatory and antimicrobial responses. These responses are particularly important in the gastrointestinal tract, where the needs for efficient nutrient uptake and host defense collide. Many pathogens have evolved to specifically colonize the intestine, causing millions of cases of enteric infections a year. A paradigm of an enteric pathogen is Salmonella enterica, a gram-negative bacterium that causes a wide range of gastrointestinal and systemic diseases. Infections with Salmonella enterica serovar Typhimurium (S. typhimurium) lead to an acute intestinal inflammation in human and animal hosts, as a result of the bacterium invading the mucosa. A distinctive feature of Salmonella is that it has not only adapted to survive in a strong inflammatory environment, but it also uses this adaptation as a strategy to gain a growth advantage over the intestinal microbiota. We will use the model organism S. typhimurium to discuss the innate immune mechanisms employed by the mammalian gastrointestinal system and how the pathogen responds and subverts these mechanisms. In particular, we focus on the recognition of extra- and intra-cellular Salmonellae by germline-encoded pattern recognition receptors of the TLR and NLR families, and how Salmonella might profit from the activation of these receptors.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
