Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei
- PMID: 22198670
- PMCID: PMC3268051
- DOI: 10.1038/nature10732
Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei
Abstract
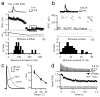
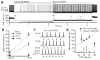
An unusual feature of the cerebellar cortex is that its output neurons, Purkinje cells, release GABA (γ-aminobutyric acid). Their high intrinsic firing rates (50 Hz) and extensive convergence predict that their target neurons in the cerebellar nuclei would be largely inhibited unless Purkinje cells pause their spiking, yet Purkinje and nuclear neuron firing rates do not always vary inversely. One indication of how these synapses transmit information is that populations of Purkinje neurons synchronize their spikes during cerebellar behaviours. If nuclear neurons respond to Purkinje synchrony, they may encode signals from subsets of inhibitory inputs. Here we show in weanling and adult mice that nuclear neurons transmit the timing of synchronous Purkinje afferent spikes, owing to modest Purkinje-to-nuclear convergence ratios (∼40:1), fast inhibitory postsynaptic current kinetics (τ(decay) = 2.5 ms) and high intrinsic firing rates (∼90 Hz). In vitro, dynamically clamped asynchronous inhibitory postsynaptic potentials mimicking Purkinje afferents suppress nuclear cell spiking, whereas synchronous inhibitory postsynaptic potentials entrain nuclear cell spiking. With partial synchrony, nuclear neurons time-lock their spikes to the synchronous subpopulation of inputs, even when only 2 out of 40 afferents synchronize. In vivo, nuclear neurons reliably phase-lock to regular trains of molecular layer stimulation. Thus, cerebellar nuclear neurons can preferentially relay the spike timing of synchronized Purkinje cells to downstream premotor areas.
Figures


Comment in
-
Neuroscience: Spikes timed through inhibition.Nature. 2012 Jan 25;481(7382):446-7. doi: 10.1038/481446a. Nature. 2012. PMID: 22281586 Free PMC article.
References
-
- Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31:785–97. - PubMed
-
- Chan-Palay V. Organization, cytology, and transmitters. Springer; Berlin: 1977. Cerebellar dentate nucleus.
-
- Palkovits M, Mezeky E, Hamori J, Szentagothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Exp Brain Res. 1977;28:189–209. - PubMed
-
- McDevitt CJ, Ebner TJ, Bloedel JR. Relationships between simultaneously recorded Purkinje cells and nuclear neurons. Brain Res. 1987;425:1–13. - PubMed
-
- Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32(6):1044–1055. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

