A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects
- PMID: 22198767
- PMCID: PMC3258628
- DOI: 10.1073/pnas.1107811108
A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects
Abstract
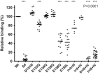
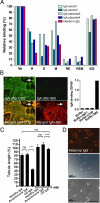
The multifunctional, protein cross-linking transglutaminase 2 (TG2) is the main autoantigen in celiac disease, an autoimmune disorder with defined etiology. Glutamine-rich gliadin peptides from ingested cereals, after their deamidation by TG2, induce T-lymphocyte activation accompanied by autoantibody production against TG2 in 1-2% of the population. The pathogenic role and exact binding properties of these antibodies to TG2 are still unclear. Here we show that antibodies from different celiac patients target the same conformational TG2 epitope formed by spatially close amino acids of adjacent domains. Glu153 and 154 on the first alpha-helix of the core domain and Arg19 on first alpha-helix of the N-terminal domain determine the celiac epitope that is accessible both in the closed and open conformation of TG2 and dependent on the relative position of these helices. Met659 on the C-terminal domain also can cooperate in antibody binding. This composite epitope is disease-specific, recognized by antibodies derived from celiac tissues and associated with biological effects when passively transferred from celiac mothers into their newborns. These findings suggest that celiac antibodies are produced in a surface-specific way for which certain homology of the central glutamic acid residues of the TG2 epitope with deamidated gliadin peptides could be a structural basis. Monoclonal mouse antibodies with partially overlapping epitope specificity released celiac antibodies from patient tissues and antagonized their harmful effects in cell culture experiments. Such antibodies or similar specific competitors will be useful in further functional studies and in exploring whether interference with celiac antibody actions leads to therapeutic benefits.
Conflict of interest statement
Conflict of interest statement: European patent applications PCT/HU2010/000036 and PCT/IB2010/000742 have been filed based on the results of this work.
Figures




Similar articles
-
Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process.J Biol Chem. 2002 Sep 13;277(37):34109-16. doi: 10.1074/jbc.M204521200. Epub 2002 Jul 1. J Biol Chem. 2002. PMID: 12093810
-
Efficient T cell-B cell collaboration guides autoantibody epitope bias and onset of celiac disease.Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15134-15139. doi: 10.1073/pnas.1901561116. Epub 2019 Jul 8. Proc Natl Acad Sci U S A. 2019. PMID: 31285344 Free PMC article.
-
Anti-type 2 transglutaminase antibodies as modulators of type 2 transglutaminase functions: a possible pathological role in celiac disease.Cell Mol Life Sci. 2018 Nov;75(22):4107-4124. doi: 10.1007/s00018-018-2902-0. Epub 2018 Aug 22. Cell Mol Life Sci. 2018. PMID: 30136165 Free PMC article. Review.
-
Activity-regulating structural changes and autoantibody epitopes in transglutaminase 2 assessed by hydrogen/deuterium exchange.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17146-51. doi: 10.1073/pnas.1407457111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404341 Free PMC article.
-
Transglutaminase 2 and Transglutaminase 2 Autoantibodies in Celiac Disease: a Review.Clin Rev Allergy Immunol. 2019 Aug;57(1):23-38. doi: 10.1007/s12016-016-8557-4. Clin Rev Allergy Immunol. 2019. PMID: 27263022 Review.
Cited by
-
Intestinal-mucosa anti-transglutaminase antibody assays to test for genetic gluten intolerance.Cell Mol Immunol. 2014 Nov;11(6):617-20. doi: 10.1038/cmi.2014.32. Epub 2014 Apr 28. Cell Mol Immunol. 2014. PMID: 24769794 Free PMC article. Clinical Trial. No abstract available.
-
RhoB is associated with the anti-angiogenic effects of celiac patient transglutaminase 2-targeted autoantibodies.J Mol Med (Berl). 2012 Jul;90(7):817-26. doi: 10.1007/s00109-011-0853-0. Epub 2012 Jan 6. J Mol Med (Berl). 2012. PMID: 22223195
-
Antibody biomarker discovery through in vitro directed evolution of consensus recognition epitopes.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19330-5. doi: 10.1073/pnas.1314792110. Epub 2013 Nov 12. Proc Natl Acad Sci U S A. 2013. PMID: 24222690 Free PMC article.
-
Gluten-Induced Extra-Intestinal Manifestations in Potential Celiac Disease-Celiac Trait.Nutrients. 2019 Feb 1;11(2):320. doi: 10.3390/nu11020320. Nutrients. 2019. PMID: 30717318 Free PMC article. Review.
-
Structures of Human Transglutaminase 2: Finding Clues for Interference in Cross-linking Mediated Activity.Int J Mol Sci. 2020 Mar 23;21(6):2225. doi: 10.3390/ijms21062225. Int J Mol Sci. 2020. PMID: 32210142 Free PMC article. Review.
References
-
- Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. - PubMed
-
- Hang J, Zemskov EA, Lorand L, Belkin AM. Identification of a novel recognition sequence for fibronectin within the NH2-terminal-sandwich domain of tissue transglutaminase. J Biol Chem. 2005;280:23675–23683. - PubMed
-
- Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–525. - PubMed
-
- Halttunen T, Mäki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology. 1999;116:566–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

