IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs
- PMID: 22198948
- PMCID: PMC3262877
- DOI: 10.4049/jimmunol.1102832
IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs
Abstract
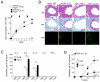
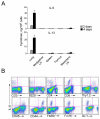
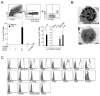
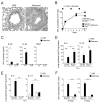
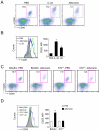
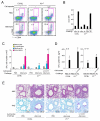
Innate immunity provides the first line of response to invading pathogens and a variety of environmental insults. Recent studies identified novel subsets of innate lymphoid cells that are capable of mediating immune responses in mucosal organs. In this paper, we describe a subset of lymphoid cells that is involved in innate type 2 immunity in the lungs. Airway exposure of naive BALB/c or C57BL/6J mice to IL-33 results in a rapid (<12 h) production of IL-5 and IL-13 and marked airway eosinophilia independently of adaptive immunity. In the lungs of nonsensitized naive mice, IL-33-responsive cells were identified that have a lymphoid morphology, lack lineage markers, highly express CD25, CD44, Thy1.2, ICOS, Sca-1, and IL-7Rα (i.e., Lin(-)CD25(+)CD44(hi) lymphoid cells), and require IL-7Rα for their development. Airway exposure of naive mice to a clinically relevant ubiquitous fungal allergen, Alternaria alternata, increases bronchoalveolar lavage levels of IL-33, followed by IL-5 and IL-13 production and airway eosinophilia without T or B cells. This innate type 2 response to the allergen is nearly abolished in mice deficient in IL-33R (i.e., ST2), and the Lin(-)CD25(+)CD44(hi) lymphoid cells in the lungs are required and sufficient to mediate the response. Thus, a subset of innate immune cells that responds to IL-33 and vigorously produces Th2-type cytokines is present in mouse lungs. These cells may provide a novel mechanism for type 2 immunity in the airways and induction of allergic airway diseases such as asthma.
Figures

References
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

