Characterization of ocular surface epithelial and progenitor cell markers in human adipose stromal cells derived from lipoaspirates
- PMID: 22199247
- PMCID: PMC3292382
- DOI: 10.1167/iovs.11-7550
Characterization of ocular surface epithelial and progenitor cell markers in human adipose stromal cells derived from lipoaspirates
Abstract
Purpose: The goal of this study was to characterize and compare mesenchymal stem cells from adult human adipose tissue (ADS cells) with progenitor cell lines from the human corneoscleral limbus and to analyze their potential for the expression of epithelial markers.
Methods: Stem cell markers (CD34, CD90, p63, and ABCG2) and epithelial cell markers (CK3/76, CK12, CK76, CK19, and CK1/5/10/14) were analyzed by immunostaining, flow cytometry, Western blot analysis, and PCR methods. The authors assayed adhesion and proliferation on different extracellular matrix proteins.
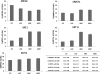
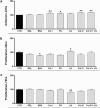
Results: ADS cells expressed a set of progenitor cell markers, including p63 and ABCG2. CK12 expression in ADS cell cultures increased spontaneously and progressively by differential adhesion, which demonstrates the cells' potential and capability to acquire epithelial-like cell characteristics. The authors observed an increase in the adhesion and proliferation of ADS cells seeded onto different basement membrane extracellular matrix proteins. Laminin substrates reduced the proliferative state of ADS cells.
Conclusions: The expression of putative stem cell markers (CD90, ABCG2, and p63) and cytokeratins (CK12 and CK76) supports the hypothesis that ADS cells have self-renewal capacity and intrinsic plasticity that enables them to acquire some epithelial-like characteristics. Therefore, adult ADS cells could be a potential source for cell therapy in ocular surface regeneration.
Figures





Similar articles
-
Mesenchymal cells from limbal stroma of human eye.Mol Vis. 2008 Mar 4;14:431-42. Mol Vis. 2008. PMID: 18334960 Free PMC article.
-
Ex vivo expanded SSEA-4+ human limbal stromal cells are multipotent and do not express other embryonic stem cell markers.Mol Vis. 2012;18:1289-300. Epub 2012 May 14. Mol Vis. 2012. PMID: 22665977 Free PMC article.
-
Ex vivo human corneal epithelial cell expansion from a xeno-feeder-free system.Ophthalmic Res. 2015;53(4):217-24. doi: 10.1159/000375110. Epub 2015 Apr 18. Ophthalmic Res. 2015. PMID: 25896314
-
Limbal stem cells: the search for a marker.Clin Exp Ophthalmol. 2006 Jan-Feb;34(1):64-73. doi: 10.1111/j.1442-9071.2006.01147.x. Clin Exp Ophthalmol. 2006. PMID: 16451261 Review.
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Priming human adipose-derived mesenchymal stem cells for corneal surface regeneration.J Cell Mol Med. 2021 Jun;25(11):5124-5137. doi: 10.1111/jcmm.16501. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33951289 Free PMC article.
-
Limbal Stem Cells from Aged Donors Are a Suitable Source for Clinical Application.Stem Cells Int. 2016;2016:3032128. doi: 10.1155/2016/3032128. Epub 2016 Nov 30. Stem Cells Int. 2016. PMID: 28042298 Free PMC article.
-
Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn.Eye Contact Lens. 2014 Jul;40(4):243-7. doi: 10.1097/ICL.0000000000000045. Eye Contact Lens. 2014. PMID: 24901976 Free PMC article.
-
Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Corneal Epithelial Repair In Vitro.Cells. 2021 May 19;10(5):1254. doi: 10.3390/cells10051254. Cells. 2021. PMID: 34069578 Free PMC article.
-
Effect of photoperiod on the feline adipose transcriptome as assessed by RNA sequencing.BMC Vet Res. 2014 Jul 3;10:146. doi: 10.1186/1746-6148-10-146. BMC Vet Res. 2014. PMID: 24992939 Free PMC article. Clinical Trial.
References
-
- Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3:141–157 - PubMed
-
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425 - PubMed
-
- Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

