Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function
- PMID: 22199367
- PMCID: PMC3288772
- DOI: 10.1161/ATVBAHA.111.243121
Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function
Abstract
Objective: Age-related aortic stiffness is an independent risk factor for cardiovascular diseases. Although oxidative stress is implicated in aortic stiffness, the underlying molecular mechanisms remain unelucidated. Here, we examined the source of oxidative stress in aging and its effect on smooth muscle cell (SMC) function and aortic compliance using mutant mouse models.
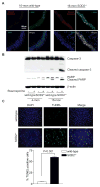
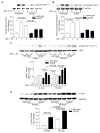
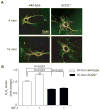
Methods and results: Pulse wave velocity, determined using Doppler, increased with age in superoxide dismutase 2 (SOD2)+/- but not in wild-type, p47phox-/- and SOD1+/- mice. Echocardiography showed impaired cardiac function in these mice. Increased collagen I expression, impaired elastic lamellae integrity, and increased medial SMC apoptosis were observed in the aortic wall of aged SOD2+/- versus wild-type (16-month-old) mice. Aortic SMCs from aged SOD2+/- mice showed increased collagen I and decreased elastin expression, increased matrix metalloproteinase-2 expression and activity, and increased sensitivity to staurosporine-induced apoptosis versus aged wild-type and young (4-month-old) SOD2+/- mice. Smooth muscle α-actin levels were increased with age in SOD2+/- versus wild-type SMCs. Aged SOD2+/- SMCs had attenuated insulin-like growth factor-1-induced Akt and Forkhead box O3a phosphorylation and prolonged tumor necrosis factor-α-induced Jun N-terminal kinase 1 activation. Aged SOD2+/- SMCs had increased mitochondrial superoxide but decreased hydrogen peroxide levels. Finally, dominant-negative Forkhead box O3a overexpression attenuated staurosporine-induced apoptosis in aged SOD2+/- SMCs.
Conclusion: Mitochondrial oxidative stress over a lifetime causes aortic stiffening, in part by inducing vascular wall remodeling, intrinsic changes in SMC stiffness, and aortic SMC apoptosis.
Figures



References
-
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. - PubMed
-
- Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206. - PubMed
-
- Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart. 2010;96:817–823. - PubMed
-
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

