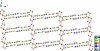Diaqua-bis-(4,4'-bipyridine-κN)bis-(2,4,5-trifluoro-3-hy-droxy-benzoato-κO)manganese(II)
- PMID: 22199603
- PMCID: PMC3238726
- DOI: 10.1107/S1600536811049610
Diaqua-bis-(4,4'-bipyridine-κN)bis-(2,4,5-trifluoro-3-hy-droxy-benzoato-κO)manganese(II)
Abstract
In the title compound, [Mn(C(7)H(2)F(3)O(3))(2)(C(10)H(8)N(2))(2)(H(2)O)(2)], the Mn(II) ion, situated on a centre of inversion, has a distorted octa-hedral coordination geometry and is coordinated by two N atoms from two 4,4'-bipyridine ligands, two O atoms from two 2,4,5-trifluoro-3-hy-droxy-benzoate ligands and two water mol-ecules. Inter-molecular O-H⋯N hydrogen bonds link the mol-ecules into a chain along the a axis. Inter-actions between neighboring chains occur through O-H⋯O hydrogen bonds, which link the chains into a two-dimensional supra-molecular network parallel to the ac plane. In addition, O-H⋯O hydrogen bonds between the water mol-ecules and carboxyl-ate groups also exist in the the crystal structure.
Figures
References
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Gielen, M., Boualam, M., Meriem, A., Mahieu, B., Biesemans, M. & Willem, R. (1992). Heteroat. Chem. 3, 449–452.
-
- Ma, C. L., Sun, J. S. & Zhang, R. F. (2006). J. Organomet. Chem. 691, 5885–5898.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Shi, Z. Q., Ji, N. N., Zhao, R. G., Li, J. K. & Li, Z. F. (2011). Struct. Chem. 22, 225–233.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous