Interleukin-1 beta and neurotrophin-3 synergistically promote neurite growth in vitro
- PMID: 22200088
- PMCID: PMC3275552
- DOI: 10.1186/1742-2094-8-183
Interleukin-1 beta and neurotrophin-3 synergistically promote neurite growth in vitro
Abstract
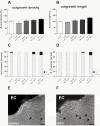
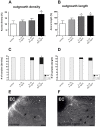
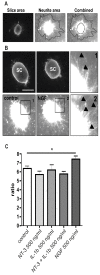
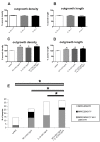
Pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) are considered to exert detrimental effects during brain trauma and in neurodegenerative disorders. Consistently, it has been demonstrated that IL-1β suppresses neurotrophin-mediated neuronal cell survival rendering neurons vulnerable to degeneration. Since neurotrophins are also well known to strongly influence axonal plasticity, we investigated here whether IL-1β has a similar negative impact on neurite growth. We analyzed neurite density and length of organotypic brain and spinal cord slice cultures under the influence of the neurotrophins NGF, BDNF, NT-3 and NT-4. In brain slices, only NT-3 significantly promoted neurite density and length. Surprisingly, a similar increase of neurite growth was induced by IL-1β. Additionally, both factors increased the number of brain slices displaying maximal neurite growth. Furthermore, the co-administration of IL-1β and NT-3 significantly increased the number of brain slices displaying maximal neurite growth compared to single treatments. These data indicate that these two factors synergistically stimulate two distinct aspects of neurite outgrowth, namely neurite density and neurite length from acute organotypic brain slices.
Figures
References
-
- Bauer J, Berkenbosch F, Van Dam AM, Dijkstra CD. Demonstration of interleukin-1 beta in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. Journal of neuroimmunology. 1993;48(1):13–21. doi: 10.1016/0165-5728(93)90053-2. - DOI - PubMed
-
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16(5):932–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

