Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice
- PMID: 22200503
- PMCID: PMC4096697
- DOI: 10.1016/j.vaccine.2011.12.064
Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice
Abstract
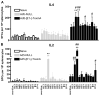
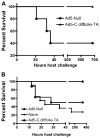
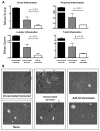
Clostridium difficile associated diarrhea (CDAD) is a critical public health problem worldwide with over 300,000 cases every year in the United States alone. Clearly, a potent vaccine preventing the morbidity and mortality caused by this detrimental pathogen is urgently required. However, vaccine efforts to combat C. difficile infections have been limited both in scope as well as to efficacy, as such there is not a vaccine approved for use against C. difficile to date. In this study, we have used a highly potent Adenovirus (Ad) based platform to create a vaccine against C. difficile. The Ad-based vaccine was able to generate rapid and robust humoral as well as cellular (T-cell) immune responses in mice that correlated with provision of 100% protection from lethal challenge with C. difficile toxin A. Most relevant to the clinical utility of this vaccine formulation was our result that toxin A specific IgGs were readily detected in plasma of Ad immunized mice as early as 3 days post vaccination. In addition, we found that several major immuno-dominant T cell epitopes were identified in toxin A, suggesting that the role of the cellular arm in protection from C. difficile infections may be more significant than previously appreciated. Therefore, our studies confirm that an Adenovirus based-C. difficile vaccine could be a promising candidate for prophylactic vaccination both for use in high risk patients and in high-risk environments.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


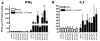
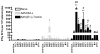

References
-
- Pechine S, Janoir C, Boureau H, Gleizes A, Tsapis N, Hoys S, et al. Diminished intestinal colonization by Clostridium difficile and immune response in mice after mucosal immunization with surface proteins of Clostridium difficile. Vaccine. 2007;25(May 20):3946–54. - PubMed
-
- Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(September 9):549–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

