Interferon Regulatory Factor 1 (IRF-1) induces p21(WAF1/CIP1) dependent cell cycle arrest and p21(WAF1/CIP1) independent modulation of survivin in cancer cells
- PMID: 22200613
- PMCID: PMC3304016
- DOI: 10.1016/j.canlet.2011.12.027
Interferon Regulatory Factor 1 (IRF-1) induces p21(WAF1/CIP1) dependent cell cycle arrest and p21(WAF1/CIP1) independent modulation of survivin in cancer cells
Abstract
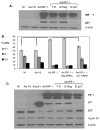
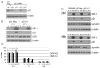
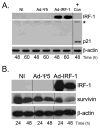
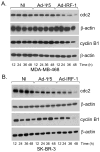
We have shown that the ectopic expression of Interferon Regulatory Factor 1 (IRF-1) results in human cancer cell death accompanied by the down-regulation of the Inhibitor of Apoptosis Protein (IAP) survivin and the induction of the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). In this report, we investigated the direct role of p21 in the suppression of survivin. We show that IRF-1 down-regulates cyclin B1, cdc-2, cyclin E, E2F1, Cdk2, Cdk4, and results in p21-mediated G1 cell cycle arrest. Interestingly, while p21 directly mediates G1 cell cycle arrest, IRF-1 or other IRF-1 signaling pathways may directly regulate survivin in human cancer cells.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nat. 1988;391:700–703. - PubMed
-
- Yim JH, Wu SJ, Casey MJ, Norton JA, Doherty GM. IFN regulatory factor-1 gene transfer into an aggressive, nonimmunogenic sarcoma suppresses the malignant phenotype and enhances immunogenicity in syngeneic mice. J Immunol. 1997;158:1284–1292. - PubMed
-
- Yim JH, Wu SJ, Lowney JK, Vander Velde TL, Doherty GM. Enhancing in vivo tumorigenicity of B16 melanoma by overexpressing interferon regulatory factor-2: resistance to endogenous IFN-gamma. J Interferon Cytokine Res. 1999;19:723–729. - PubMed
-
- Kim PK, Armstrong M, Liu Y, Yan P, Bucher B, Zuckerbraun BS, Gambotto A, Billiar TR, Yim JH. IRF-1 expression induces apoptosis and inhibits tumor growth in mouse mammary cancer cells in vitro and in vivo. Oncogene. 2004;23:1125–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

