Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina
- PMID: 22200614
- PMCID: PMC3349878
- DOI: 10.1016/j.ajpath.2011.11.006
Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina
Abstract
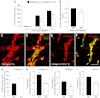
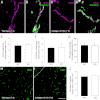
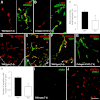
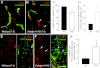
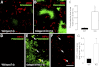
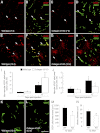
To investigate the importance of the vascular basal lamina in tumor blood vessel morphogenesis and function, we compared vessel development, vessel function, and progression of B16F10 melanoma tumors in the brains of wild-type and collagen VI-null mice. In 7-day tumors in the absence of collagen VI, the width of the vascular basal lamina was reduced twofold. Although the ablation of collagen VI did not alter the abundance of blood vessels, a detailed analysis of the number of either pericytes or endothelial cells (or pericyte coverage of endothelial cells) showed that collagen VI-dependent defects during the assembly of the basal lamina have negative effects on both pericyte maturation and the sprouting and survival of endothelial cells. As a result of these deficits, vessel patency was reduced by 25%, and vessel leakiness was increased threefold, resulting in a 10-fold increase in tumor hypoxia along with a fourfold increase in hypoxia-inducible factor-1α expression. In 12-day collagen VI-null tumors, vascular endothelial growth factor expression was increased throughout the tumor stroma, in contrast to the predominantly vascular pattern of vascular endothelial growth factor expression in wild-type tumors. Vessel size was correspondingly reduced in 12-day collagen VI-null tumors. Overall, these vascular deficits produced a twofold decrease in tumor volume in collagen VI-null mice, confirming that collagen VI-dependent basal lamina assembly is a critical aspect of vessel development.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse.Dev Biol. 2010 Aug 15;344(2):1035-46. doi: 10.1016/j.ydbio.2010.06.023. Epub 2010 Jun 27. Dev Biol. 2010. PMID: 20599895 Free PMC article.
-
Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan.Breast Cancer Res. 2012 Apr 24;14(2):R67. doi: 10.1186/bcr3174. Breast Cancer Res. 2012. PMID: 22531600 Free PMC article.
-
The NG2 Proteoglycan in Pericyte Biology.Adv Exp Med Biol. 2018;1109:5-19. doi: 10.1007/978-3-030-02601-1_2. Adv Exp Med Biol. 2018. PMID: 30523586 Review.
-
NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function.Angiogenesis. 2014 Jan;17(1):61-76. doi: 10.1007/s10456-013-9378-1. Epub 2013 Aug 8. Angiogenesis. 2014. PMID: 23925489 Free PMC article.
-
NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression.Microcirculation. 2016 Feb;23(2):122-33. doi: 10.1111/micc.12251. Microcirculation. 2016. PMID: 26465118 Free PMC article. Review.
Cited by
-
Gold Nanoparticles Disrupt the IGFBP2/mTOR/PTEN Axis to Inhibit Ovarian Cancer Growth.Adv Sci (Weinh). 2022 Nov;9(31):e2200491. doi: 10.1002/advs.202200491. Epub 2022 Sep 14. Adv Sci (Weinh). 2022. PMID: 36104215 Free PMC article.
-
The Extracellular Matrix Environment of Clear Cell Renal Cell Carcinoma.Cancers (Basel). 2022 Aug 23;14(17):4072. doi: 10.3390/cancers14174072. Cancers (Basel). 2022. PMID: 36077607 Free PMC article. Review.
-
Glioblastoma niches: from the concept to the phenotypical reality.Neurol Sci. 2018 Jul;39(7):1161-1168. doi: 10.1007/s10072-018-3408-0. Epub 2018 May 8. Neurol Sci. 2018. PMID: 29736738 Review.
-
Angiogenesis and Blood-Brain Barrier Permeability in Vascular Remodeling after Stroke.Curr Neuropharmacol. 2020;18(12):1250-1265. doi: 10.2174/1570159X18666200720173316. Curr Neuropharmacol. 2020. PMID: 32691713 Free PMC article.
-
Pericyte-endothelial cell interaction: a survival mechanism for the tumor vasculature.Cell Adh Migr. 2012 May-Jun;6(3):157-9. doi: 10.4161/cam.20252. Epub 2012 May 1. Cell Adh Migr. 2012. PMID: 22568989 Free PMC article. No abstract available.
References
-
- Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. - PubMed
-
- Davis G.E., Senger D.R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. - PubMed
-
- Gaengel K., Genové G., Armulik A., Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

