Inflammation-induced lymph node lymphangiogenesis is reversible
- PMID: 22200615
- PMCID: PMC3349879
- DOI: 10.1016/j.ajpath.2011.11.010
Inflammation-induced lymph node lymphangiogenesis is reversible
Abstract
The extent of lymph node metastasis is a prognostic indicator of disease progression in many malignancies. Current noninvasive imaging technologies for the clinical assessment of lymph node metastases are based on the detection of cancer cells and commonly suffer from a lack of sensitivity. Recent evidence has indicated that the expansion of lymphatic networks (ie, lymphangiogenesis) within tumor-draining lymph nodes might be the earliest sign of metastasis. Therefore, we recently developed a noninvasive imaging method to visualize lymph node lymphangiogenesis in mice using radiolabeled antibodies against the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) as well as positron emission tomography (PET). This technique, termed anti-LYVE-1 immuno-PET, was found to be very sensitive in the detection of metastasis to the lymph nodes. However, lymphatic vessel expansion to the lymph nodes can also be induced by inflammation, and it is currently unclear whether such vessel expansion is reversed once inflammation has resolved. Detection of residual inflammation-induced lymph node lymphangiogenesis, thus, might hamper the identification of metastasized lymph nodes. In this study, we therefore used a well-established mouse model of inflammation in the skin to investigate whether lymphatic vessels in the lymph nodes regress on resolution of inflammation. Our data reveal that the lymphatic network indeed regresses on the resolution of inflammation and that we can image this process by anti-LYVE-1 immuno-PET.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

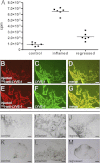
Similar articles
-
In vivo imaging of inflammation- and tumor-induced lymph node lymphangiogenesis by immuno-positron emission tomography.Cancer Res. 2010 Nov 1;70(21):8842-51. doi: 10.1158/0008-5472.CAN-10-0896. Epub 2010 Oct 26. Cancer Res. 2010. PMID: 20978206 Free PMC article.
-
In vivo imaging of lymph node lymphangiogenesis by immuno-positron emission tomography.Methods Mol Biol. 2013;961:129-40. doi: 10.1007/978-1-62703-227-8_6. Methods Mol Biol. 2013. PMID: 23325639
-
124I-Labeled antibody against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1).2011 Mar 22 [updated 2011 Jul 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Mar 22 [updated 2011 Jul 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21755633 Free Books & Documents. Review.
-
Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF-C as the earliest premetastatic indicator.Int J Oncol. 2012 Dec;41(6):2073-8. doi: 10.3892/ijo.2012.1665. Epub 2012 Oct 16. Int J Oncol. 2012. PMID: 23076721 Free PMC article.
-
AlexaFluor 488-conjugated antibody against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1).2011 Mar 24 [updated 2011 Jul 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Mar 24 [updated 2011 Jul 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21755634 Free Books & Documents. Review.
Cited by
-
Inflammation-associated lymphangiogenesis: a double-edged sword?J Clin Invest. 2014 Mar;124(3):936-42. doi: 10.1172/JCI71607. Epub 2014 Mar 3. J Clin Invest. 2014. PMID: 24590279 Free PMC article. Review.
-
The lymphatics in kidney health and disease.Nat Rev Nephrol. 2021 Oct;17(10):655-675. doi: 10.1038/s41581-021-00438-y. Epub 2021 Jun 22. Nat Rev Nephrol. 2021. PMID: 34158633 Review.
-
Lymphatic Vessel Regression and Its Therapeutic Applications: Learning From Principles of Blood Vessel Regression.Front Physiol. 2022 Mar 22;13:846936. doi: 10.3389/fphys.2022.846936. eCollection 2022. Front Physiol. 2022. PMID: 35392370 Free PMC article. Review.
-
Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation's fire?Exp Biol Med (Maywood). 2017 Apr;242(8):884-895. doi: 10.1177/1535370217697385. Epub 2017 Mar 7. Exp Biol Med (Maywood). 2017. PMID: 28346012 Free PMC article. Review.
-
Tube-like structures with co-expression of D2-40 and CD34: newly formed vasculatures?Int J Biol Sci. 2012;8(8):1206-16. doi: 10.7150/ijbs.5147. Epub 2012 Oct 19. Int J Biol Sci. 2012. PMID: 23136548 Free PMC article.
References
-
- Jaffer F.A., Weissleder R. Molecular imaging in the clinical arena. Jama. 2005;293:855–862. - PubMed
-
- Qian C.N., Berghuis B., Tsarfaty G., Bruch M., Kort E.J., Ditlev J., Tsarfaty I., Hudson E., Jackson D.G., Petillo D., Chen J., Resau J.H., Teh B.T. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–10376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

