Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds
- PMID: 22200664
- PMCID: PMC3295380
- DOI: 10.1093/jxb/err336
Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds
Abstract
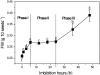
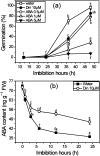
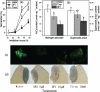
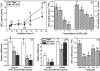
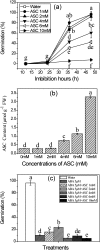
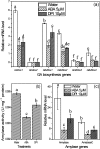
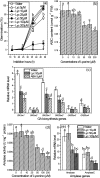
The antagonism between abscisic acid (ABA) and gibberellin (GA) plays a key role in controlling seed germination, but the mechanism of antagonism during this process is not known. The possible links among ABA, reactive oxygen species (ROS), ascorbic acid (ASC), and GA during rice seed germination were investigated. Unlike in non-seed tissues where ROS production is increased by ABA, ABA reduced ROS production in imbibed rice seeds, especially in the embryo region. Such reduced ROS also led to an inhibition of ASC production. GA accumulation was also suppressed by a reduced ROS and ASC level, which was indicated by the inhibited expression of GA biosynthesis genes, amylase genes, and enzyme activity. Application of exogenous ASC can partially rescue seed germination from ABA treatment. Production of ASC, which acts as a substrate in GA biosynthesis, was significantly inhibited by lycorine which thus suppressed the accumulation of GA. Consequently, expression of GA biosynthesis genes was suppressed by the low levels of ROS and ASC in ABA-treated seeds. It can be concluded that ABA regulates seed germination in multiple dimensions. ROS and ASC are involved in its inhibition of GA biosynthesis.
Figures



References
-
- Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. - PubMed
-
- Arrigoni O, Arrigoni-Liso R, Calabrese G. Lycorine as an inhibitor of ascorbic acid biosynthesis. Nature. 1975;256:513–514.
-
- Arrigoni O, De Gara L, Paciolla C, Evidente A, De Pinto MC, Liso R. Lycorine: a powerful inhibitor of L-galactono-γ-lactone dehydrogenase activity. Journalof Plant Physiology. 1997;150:362–364.
-
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta. 2002;1569:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

