The light-sensitive conductance of melanopsin-expressing Joseph and Hesse cells in amphioxus
- PMID: 22200946
- PMCID: PMC3250099
- DOI: 10.1085/jgp.201110717
The light-sensitive conductance of melanopsin-expressing Joseph and Hesse cells in amphioxus
Abstract
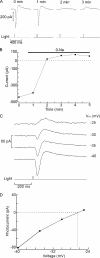
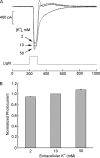
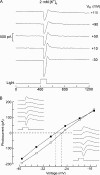
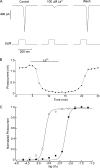
Two types of microvillar photoreceptors in the neural tube of amphioxus, an early chordate, sense light via melanopsin, the same photopigment as in "circadian" light detectors of higher vertebrates. Because in amphioxus melanopsin activates a G(q)/phospholipase C cascade, like phototransduction in arthropods and mollusks, possible commonalities in the photoconductance were investigated. Unlike other microvillar photoreceptors, reversal of the photocurrent can only be attained upon replacement of extracellular Na(+). In addition to Na(+), Ca(2+) is also permeant, as indicated by the fact that (a) in normal ionic conditions the photocurrent remains inward at V(m) > E(Na); (b) in Na-free solution a small residual inward photocurrent persists at V(m) near resting level, provided that Ca is present; and (c) V(rev) exhibits a modest shift with [Ca](o) manipulations. The unusual reversal is accounted for by an uncommonly low permeability of the light-dependent channels to K(+), as [K](o) only marginally affects the photocurrent amplitude and its reversal. Lanthanum and ruthenium red (RuR), two TRP channel antagonists, reversibly suppress the response to photostimulation of moderate intensity; therefore, the melanopsin-initiated cascade may recruit ion channels of the same family as those of rhabdomeric photoreceptors. With brighter lights, blockage declines, so that both La(3+) and RuR induce a right shift in the sensitivity curve without a reduction of its asymptote. Nonetheless, an effect on the transduction cascade, rather than the channels, was ruled out on the basis of the voltage dependency of the blockade and the lack of effects of intracellular application of the same substances. The mechanisms of action of these antagonists thus entail a state-dependent blockade, with a higher affinity for the channel in the closed conformation. Collectively, the results indicate a kinship of the light-sensitive channels of amphioxus with those of invertebrate rhabdomeric visual cells and support the representation of this lineage of photoreceptors among chordates.
Figures



Similar articles
-
Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling.J Neurosci. 2012 Dec 12;32(50):17977-87. doi: 10.1523/JNEUROSCI.3069-12.2012. J Neurosci. 2012. PMID: 23238714 Free PMC article.
-
Calcium activates the light-dependent conductance in melanopsin-expressing photoreceptors of amphioxus.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7845-50. doi: 10.1073/pnas.1420265112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056310 Free PMC article.
-
Melanopsin-expressing amphioxus photoreceptors transduce light via a phospholipase C signaling cascade.PLoS One. 2012;7(1):e29813. doi: 10.1371/journal.pone.0029813. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235344 Free PMC article.
-
Phototransduction in ganglion-cell photoreceptors.Pflugers Arch. 2007 Aug;454(5):849-55. doi: 10.1007/s00424-007-0242-2. Epub 2007 Mar 10. Pflugers Arch. 2007. PMID: 17351786 Review.
-
Melanopsin phototransduction: slowly emerging from the dark.Prog Brain Res. 2012;199:19-40. doi: 10.1016/B978-0-444-59427-3.00002-2. Prog Brain Res. 2012. PMID: 22877657 Review.
Cited by
-
Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling.J Neurosci. 2012 Dec 12;32(50):17977-87. doi: 10.1523/JNEUROSCI.3069-12.2012. J Neurosci. 2012. PMID: 23238714 Free PMC article.
-
The physiological role of TRP channels in sleep and circadian rhythm.J Cell Mol Med. 2024 May;28(9):e18274. doi: 10.1111/jcmm.18274. J Cell Mol Med. 2024. PMID: 38676362 Free PMC article. Review.
-
Calcium activates the light-dependent conductance in melanopsin-expressing photoreceptors of amphioxus.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7845-50. doi: 10.1073/pnas.1420265112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056310 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

