Transmembrane mucins as novel therapeutic targets
- PMID: 22201009
- PMCID: PMC3245640
- DOI: 10.1586/eem.11.70
Transmembrane mucins as novel therapeutic targets
Abstract
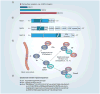
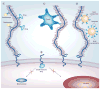
Membrane-tethered mucin glycoproteins are abundantly expressed at the apical surfaces of simple epithelia, where they play important roles in lubricating and protecting tissues from pathogens and enzymatic attack. Notable examples of these mucins are MUC1, MUC4 and MUC16 (also known as cancer antigen 125). In adenocarcinomas, apical mucin restriction is lost and overall expression is often highly increased. High-level mucin expression protects tumors from killing by the host immune system, as well as by chemotherapeutic agents, and affords protection from apoptosis. Mucin expression can increase as the result of gene duplication and/or in response to hormones, cytokines and growth factors prevalent in the tumor milieu. Rises in the normally low levels of mucin fragments in serum have been used as markers of disease, such as tumor burden, for many years. Currently, several approaches are being examined that target mucins for immunization or nanomedicine using mucin-specific antibodies.
Figures
References
-
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. - PubMed
-
- Choudhury A, Moniaux N, Ringel J, et al. Alternate splicing at the 3′-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog Carcinog Mutagen. 2001;21(1):83–96. - PubMed
-
- Wreschner DH, Hareuveni M, Tsarfaty I, et al. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur J Biochem. 1990;189(3):463–473. - PubMed
-
- Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267(9):6171–6177. - PubMed
Websites
-
- National Institutes of Health Registry of Clinical Trials. www.clinicaltrials.gov.
-
- Press release: Therion Reports Results Of Phase 3 PANVAC-VF Trial And Announces Plans For Company Sale. www.medicalnewstoday.com/releases/46137.php.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
