To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework
- PMID: 22201281
- PMCID: PMC3272484
- DOI: 10.1037/a0026673
To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework
Abstract
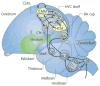
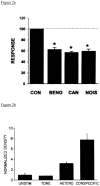
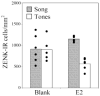
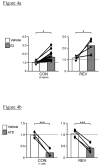
Gonadal hormones modulate behavioral responses to sexual stimuli, and communication signals can also modulate circulating hormone levels. In several species, these combined effects appear to underlie a two-way interaction between circulating gonadal hormones and behavioral responses to socially salient stimuli. Recent work in songbirds has shown that manipulating local estradiol levels in the auditory forebrain produces physiological changes that affect discrimination of conspecific vocalizations and can affect behavior. These studies provide new evidence that estrogens can directly alter auditory processing and indirectly alter the behavioral response to a stimulus. These studies show that: 1) Local estradiol action within an auditory area is necessary for socially relevant sounds to induce normal physiological responses in the brains of both sexes; 2) These physiological effects occur much more quickly than predicted by the classical time-frame for genomic effects; 3) Estradiol action within the auditory forebrain enables behavioral discrimination among socially relevant sounds in males; and 4) Estradiol is produced locally in the male brain during exposure to particular social interactions. The accumulating evidence suggests a socio-neuro-endocrinology framework in which estradiol is essential to auditory processing, is increased by a socially relevant stimulus, acts rapidly to shape perception of subsequent stimuli experienced during social interactions, and modulates behavioral responses to these stimuli. Brain estrogens are likely to function similarly in both songbird sexes because aromatase and estrogen receptors are present in both male and female forebrain. Estrogenic modulation of perception in songbirds and perhaps other animals could fine-tune male advertising signals and female ability to discriminate them, facilitating mate selection by modulating behaviors.
Figures

References
-
- Adkins-Regan E. Hormone specificity, androgen metabolism, and social behavior. American Zoologist. 1981;21:257–71.
-
- Azcotia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. - PubMed
-
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23:137–78. - PubMed

